Injecting Electrons into a Single Sheet of Carbon Atoms through Chemical Substitution
May result in transparent conductors for organic solar cells with increased power conversion efficiency
August 30, 2011
Researchers in Columbia University’s Energy Frontier Research Center, working in collaboration with scientists at the U.S. Department of Energy’s Brookhaven National Laboratory and SLAC National Accelerator Laboratory, have demonstrated that it is possible to alter the electronic structure of graphene, a one-atom-thick sheet of carbon, through the direct substitution of individual nitrogen atoms for carbon atoms. The discovery, described in the August 19, 2011, issue of Science, could enable the use of these ultrathin carbon films in such applications as transparent conductors for organic solar cells with increased power conversion efficiency.
In organic solar cells today, the active layers that convert sunlight into electricity are made from organic polymers. Researchers envision solar cells that could potentially be manufactured as easily and inexpensively as plastics. Using ultrathin carbon sheets to form the transparent electrical contact could be a big step forward for these organic solar cells, which could ultimately be attractive replacements for silicon-based solar cells — if their efficiency can be improved.
“This research represents one approach to controlling electrical conductivity in graphene layers — an essential step toward making these materials more competitive for real technology applications like organic solar cells,” said Mark Hybertsen, a theoretical physicist at Brookhaven Lab’s Center for Functional Nanomaterials (CFN) and co-author on the paper, who provided computational and theoretical analysis to support the results.
The incorporation of nitrogen atoms into the graphene film was achieved by adding nitrogen-containing ammonia gas to the gases typically used in the synthesis of graphene. Careful imaging using a scanning tunneling microscope (STM) at Columbia confirms that nitrogen atoms substitute for carbon atoms within the honeycomb graphene lattice.
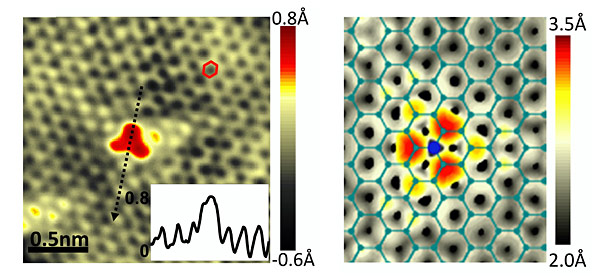
The presence of nitrogen in the scanning tunneling microscope (STM) image on the left is indicated by a red, triangular region. A theoretical simulated image (right), where the honeycomb lattice appears as an overlay and the nitrogen atom (blue) is highlighted, confirms that the nitrogen has substituted for a carbon atom in the graphene lattice.
Since each of these nitrogen atoms possesses one more electron than the carbon atom it replaces, the substitution results in a material doped with negative electrical charge. The STM measurements reveal that approximately half of the extra electronic charge resides at the nitrogen atoms, while the rest gets distributed throughout the graphene lattice.
Because the amount of injected electrical charge determines important material properties, notably the electrical conductivity, this discovery provides the means for precise control of electrical charge within this unique material. This research may therefore enable the fabrication of new devices and structures in which the electrical properties are determined through atomic substitution in a manner analogous to silicon-based electronic materials.
X-ray studies conducted at Brookhaven’s National Synchrotron Light Source and the Stanford Synchrotron Radiation Lightsource at SLAC provided additional support for the conclusions.
The CFN, one of five DOE Nanoscale Science Research Centers (NSRCs), is a collaborative partner in the Columbia University Energy Frontier Research Center through the contributions of CFN researchers Charles Black, Mark Hybertsen, and other CFN staff members. In the course of this research, Columbia scientists used the Computational Facility in the CFN to calculate the electronic properties of the nitrogen doped graphene and to simulate STM images for interpretation. Other CFN facilities are extensively used for several key projects in the Columbia University Energy Frontier Research Center.
For more information about the DOE NSRCs, please visit http://nano.energy.gov.
2011-2570 | INT/EXT | Newsroom









