Research on Platinum Nanoparticle Catalysts a Step Toward Reduced Nitrogen-Oxide Emissions in Vehicles
December 19, 2014
 enlarge
enlarge
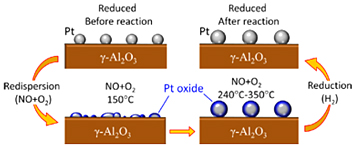
Steps in the NO to NO2 oxidation reaction, catalyzed by Pt nanoparticles. Particles tended to flatten, or redisperse, with PtO forming on their surfaces.
Stricter environmental regulations enacted in the last few years are putting a squeeze on emissions from car engines, including nitrogen oxide. While modern “lean-burn” gasoline and diesel vehicles use less fuel, they also require more oxygen. As a result, traditional three-way catalytic converters – which scrub out the nitrogen oxide, unburned hydrocarbons, and carbon monoxide – don’t adequately reduce the rate of nitrogen oxide emissions.
These circumstances are spurring researchers to investigate how to optimize the catalytic reactions that govern the storage and reduction of nitrogen oxides (NO and NO2, collectively denoted NOx) in a vehicle’s exhaust stream. These updated processes require a catalyst that performs two jobs: a storage function, often using a barium-oxide-based material as the storage medium; and an oxidation-reduction function, typically based on platinum (Pt), which converts NO to NO2 using oxygen in the air.
In this work, researchers from the University of Central Florida, Brookhaven National Laboratory (BNL) and Ruhr University Bochum in Germany studied a model catalyst system of Pt nanoparticles supported on a surface of aluminum oxide (Al2O3). Using x-rays at Brookhaven’s National Synchrotron Light Source, the group followed the evolution of the particles’ structure and chemical state during the conversion of NO to NO2, which is a crucial step in the full NOx storage and reduction reaction (of which the final products are nitrogen and oxygen). The information they learned will support additional studies aimed at finding optimal nanoscale Pt catalysts for modern lean-burn engines.
The nanoparticle samples used in this investigation were roughly the same diameter, 0.6 nanometers, but different shapes: two types were relatively flat, the third was more spherical. They were studied under “lean operation conditions,” which means that there is an excess of air as opposed to an excess of fuel. At NSLS beamline X18B, the researchers “watched” the reaction using two x-ray absorption techniques that are sensitive to the local electronic structure of the molecules in the sample. The techniques yielded specific information on the nature of the Pt-O bonds as the reaction took place.
Similar studies have been done before and have yielded significant insights into the reaction, such as key details on the evolution of the chemical state of the Pt. But these investigations could not distinguish between the different Pt-O bonds. This study, however, showed that a handful of different PtOx species formed, even at just 150 degrees Celsius, before the onset of the reaction, and were present during the entire course of the reaction, up to about 350ºC. There were Pt-O bonds that formed between the Pt and the Al2O3 substrate, Pt-O bonds from the formation of Pt oxides, and Pt-O bonds due to “chemisorbed” oxygen, in which oxygen atoms bind to the solid Pt surface, but largely leave the Pt intact.
Like previous studies, this study confirmed that size and shape do influence how well the particles act as catalysts. The more spherical nanoparticles were better at oxidizing the NO because they, themselves, were less oxidized over the course of the reaction.
“These data allowed us to distinguish between the different forms of PtO that exist during the reaction, and we concluded that it is vital to control the sizes of the nanoparticles, not just before and after the reaction but during it,” said the study’s corresponding researcher, Beatriz Roldan, a scientist at the Ruhr-University Bochum.
She continued, “Our study illustrates the dynamic changes in the structure and chemical state of nanoscale catalysts under reaction conditions, highlighting the importance of operando studies in gaining an understanding of structure-reactivity correlations.”
This research is described in the April 25, 2014, edition of the journal ACS Catalysis.
2014-5434 | INT/EXT | Newsroom