Technologies Available for License
Categories: advanced materialsenergy
2005-010: High-Activity Platinum Monolayer Fuel Cell Catalysts
Invention: 2005-010
Patent Status: U.S. Patent Number 7,855,021 was issued on December 21, 2010
For technical and licensing related questions, email tcp@bnl.gov.
Summary
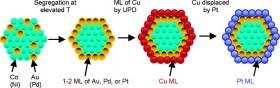
This diagram shows the process of generating the core-shell nanoparticle and coating it with a monolayer of platinum to create the high mass activity catalyst.
Platinum is the most effective and common electrocatalyst for the fuel cells commonly used in electric vehicles, but it is both rare and expensive. A new process of constructing a nanoparticle core of cheaper metals and coating it with an atomically thin layer of platinum significantly lowers costs without sacrificing catalytic activity. This custom nanoparticle design sets new standards for fuel cell cost and efficiency, with high potential for other platinum-based reactions.
Description
A new class of oxygen-reduction electrocatalysts offers high activity and very low noble metal content. They consist of Pt monolayers deposited on the surfaces of carbon-supported nonnoble metal/noble metal core- shell nanoparticles. These core-shell nanoparticles are formed by segregating the atoms of the noble metal to the nanoparticles' surfaces at elevated temperatures. A Pt monolayer is then deposited by galvanic displacement of a Cu monolayer deposited at underpotentials. The mass activity of three Pt monolayer electrocatalysts was investigated, viz., Pt/Au/Ni, Pt/Pd/Co, and Pt/Pt/Co. Each exhibited mass activity more than an order of magnitude higher than that of a state-of-the-art commercial Pt/C electrocatalyst. Geometric effects in the Pt monolayer and the effects of PtOH coverage, revealed by electrochemical data, X-ray diffraction, and X-ray absorption spectroscopy data, appear to be the source of the enhanced catalytic activity. The results demonstrate that high-activity electrocatalysts can be devised with only a fractional amount of Pt and a very small amount of another noble metal.
Benefits
These new platinum composites are "drop-in" replacements for current fuel cell electrocatalysts, offering easy incorporation in existing devices. The process used to make the electrocatalysts is, itself, an electrochemical one using benign reactants in a well-understood field and limiting reliance upon expensive materials such as platinum. Both of these factors suggest a rapid return on investment and lower overall costs to create the electrocatalysts common in proton exchange membrane (PEM) fuel cells.
Applications and Industries
These reduced-platinum electrocatalysts find their primary applications in PEM fuel cells for any kind of vehicle, although use in other reactions would be advantageous. Explorations are underway to test the performance of these structures as catalysts for emissions control, hydrogen production in hydrolyzers, and other reactions that use high levels of pure or nearly pure platinum.
Journal Publication & Intellectual Property
Contacts
-

Poornima Upadhya
Manager Technology Transfer & Commercialization
Technology Commercialization
(631) 344-4711, pupadhya@bnl.gov
-

Avijit Sen
IP Licensing & Commercialization
Technology Commercialization
(631) 344-3752, asen@bnl.gov




