Technologies Available for License
Category: biotechnology & health
2010-028: Novel CPP-115 an inactivator of GABA-AT
Invention: 2010-028
Patent Status: U.S. Patent Number 8,969,413 was issued on March 3, 2015
For technical and licensing related questions, email tcp@bnl.gov.
Summary
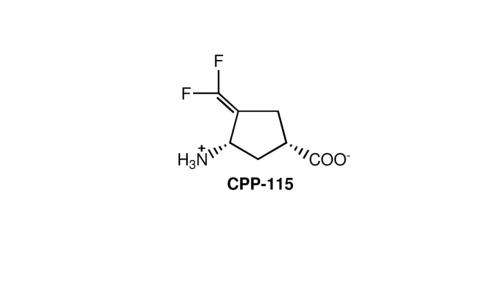
(1S,3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid
The acceptance of vigabatrin (also known as Sabril) for the treatment of both epilepsy and as a potential treatment for stimulant addiction has been hampered primarily by concerns about abnormalities of theperipheral visual field (visual field defects or VFDs) in 25-50% of patients following chronic administration of vigabatrin. Vigabatrin's well known mechanism of action is the irreversible inhibition of gamma-aminobutyric acid-aminotransferase (GABA-AT). As a result, an unmet medical need remains for a GABA-AT inhibitor with improved potency and reduced, or eliminated, visual field defects for the treatment of both addiction and epilepsy. This invention provides methods to reduce collateral damages from the visual field defects and intramyelinic edema associated with the administration of the GABA-AT inhibitor vigabatrin to a patient in need.
Description
A synthetic compound, (1S,3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), was designed as a mechanism-based inactivator of GABA-AT, which could generate a more reactive intermediate along the pathway to attachment to the active site of GABA-AT via a Michael addition. Studies showed GABA-AT inactivator (CPP-115) does not exhibit other GABAergic or off-target activities and is rapidly and completely orally absorbed and eliminated. Using in vivo microdialysis techniques in freely moving rats and micro-PET imaging techniques, CPP-115 showed similar inhibition of cocaine-induced increases in extracellular dopamine and in synaptic dopamine in the nucleus accumbens at 1/300-1/600th the dose of vigabatrin. It also blocksexpression of cocaine-induced conditioned place preference at a dose 1/300th that of vigabatrin. Overall, CPP-115 can be administered at significantly lower doses than vigabatrin, which suggests a potential new treatment for addiction with a significantly reduced risk of visual field defects.
Benefits
It is crucial that potential therapeutic compounds are selective for specific components of the GABAergic system. The principal GABAergic site of action of CPP-115 appears to be GABA-AT, the enzyme that catabolizes GABA. In addition, CPP-115 exhibits remarkable pharmacokinetic characteristics: it is not metabolized, exhibits rapid and complete oral absorption, and is rapidly eliminated, which are all highly favorable for orally delivered drugs.
Applications and Industries
CPP-115 or its pharmaceutically acceptable salts may be used to treat epilepsy, infantile spasms fibromyalgia, neuropathy, migraines related to epilepsy, restless leg syndrome, and post traumatic distress disorder at significantly lower doses that currently approved Gabaergic drugs.
Journal Publication & Intellectual Property
Contacts
-

Poornima Upadhya
Manager Technology Transfer & Commercialization
Technology Commercialization
(631) 344-4711, pupadhya@bnl.gov
-

Avijit Sen
IP Licensing & Commercialization
Technology Commercialization
(631) 344-3752, asen@bnl.gov




