- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Artificial Photosynthesis
Biomass-derived electrocatalytic composites for hydrogen evolution
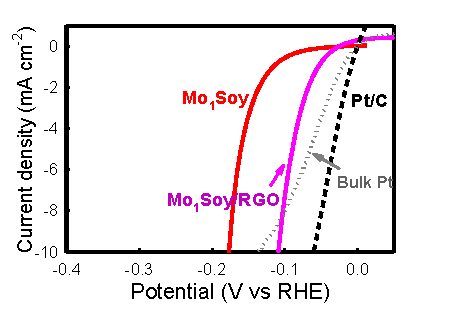 We have
developed a catalyst made from earth-abundant molybdenum and common, humble
soybeans (Mo1Soy) by a simple procedure. This catalyst, composed
of a catalytic β-Mo2C phase and an
acid-proof γ-Mo2N phase, drives the hydrogen evolution reaction
(HER) with low overpotentials, and is highly durable in a corrosive acidic
solution over a period exceeding 500 hours. When supported on graphene
sheets, the Mo1Soy catalyst exhibits very fast charge transfer
kinetics, and its performance rivals that of noble-metal catalysts such as
Pt for hydrogen production. These findings prove that the soybean (as well
as other high-protein biomass) is a useful material for the generation of
catalysts incorporating an abundant transition metal, thereby challenging
the exclusivity of platinum catalysts in hydrogen economics.
We have
developed a catalyst made from earth-abundant molybdenum and common, humble
soybeans (Mo1Soy) by a simple procedure. This catalyst, composed
of a catalytic β-Mo2C phase and an
acid-proof γ-Mo2N phase, drives the hydrogen evolution reaction
(HER) with low overpotentials, and is highly durable in a corrosive acidic
solution over a period exceeding 500 hours. When supported on graphene
sheets, the Mo1Soy catalyst exhibits very fast charge transfer
kinetics, and its performance rivals that of noble-metal catalysts such as
Pt for hydrogen production. These findings prove that the soybean (as well
as other high-protein biomass) is a useful material for the generation of
catalysts incorporating an abundant transition metal, thereby challenging
the exclusivity of platinum catalysts in hydrogen economics.
Energy Environ. Sci. 2013, 6, 1818-1826, DOI: 10.1039/C3EE40596F.




