- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Catalysis for Alternative Fuels Production
The Catalysis for Alternative Fuels Production (CAFP) group is part of the BNL catalysis science program, which advances fundamental understanding of sustainable fuel and chemical synthesis pathways with a current emphasis on conversion of CO2. The CAFP group is exploring the feasibility of producing value-added liquid products, such as aromatics and oxygenates, by developing catalytic processes in tandem with the CO2-assisted reforming and dehydrogenation of light alkanes. We combine kinetic studies using flow reactors, in-situ characterization of the electronic and structural properties of catalysts under reaction conditions, and DFT calculations of reaction pathways. Our research group at BNL Chemistry Division takes advantages of the synchrotron facilities at the National Synchrotron Light Source II (NSLS II) and the microscopy and computational facilities at the Center for Functional Nanomaterials (CFN) at BNL.
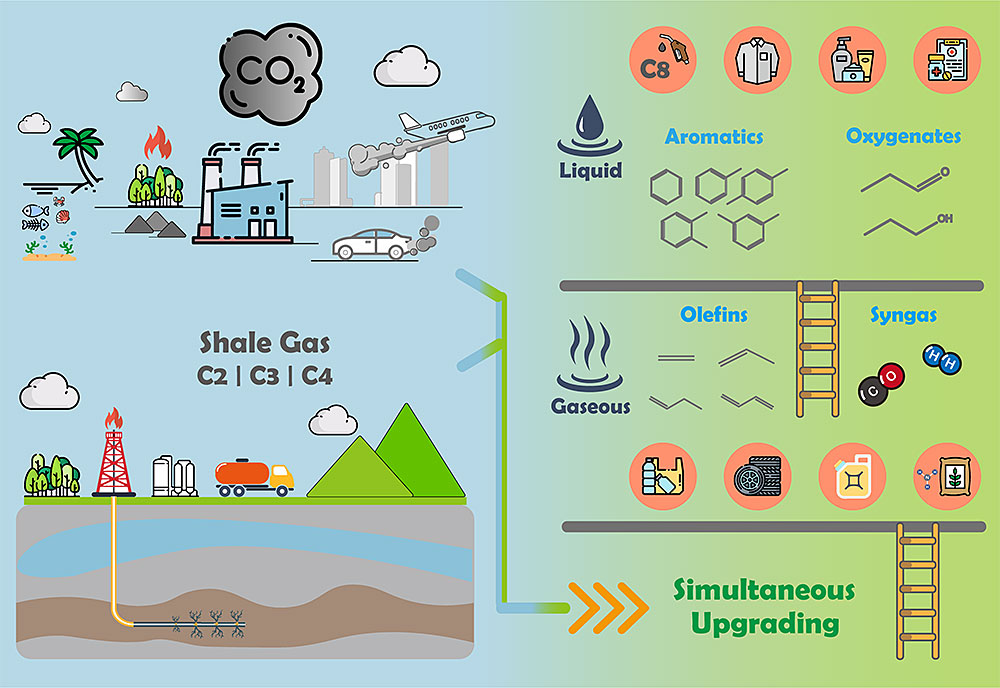
The activation of CO2 requires a dual functional catalyst: (1) the ability to adsorb/activate CO2 and (2) the availability of catalytic sites to dissociate light alkanes and to promote formation and subsequent conversion of the HxCyOz reaction intermediates leading to the dry reforming and deoxygenation pathways. For monometallic and bimetallic catalysts supported on a reducible oxide substrate, the former function is provided by the oxide and/or the metal/oxide interface, while the latter is contributed by the metal components, with bimetallic often showing better activity/selectivity over the monometallic counterparts. We have identified several classes of catalysts, especially bimetallic catalysts supported on reducible oxides, that are active and selective for the reactions of CO2 with light alkanes (ethane, propane, and butane) and are exploring their application for the simultaneous upgrading of CO2 and light alkanes. We are identifying mechanisms and descriptors to control the product selectivity (see diagram) toward dry reforming (H2 + CO) and oxidative dehydrogenation (olefin + CO + H2O) that would facilitate subsequent conversion to liquid products via either aromatization or hydroformylation reactions The diagram below shows various pathways to value-added products from CO2 reacting with alkanes.
For more information about our research, please visit Jingguang Chen’s group at Fu Foundation School of Engineering and Applied Science, Columbia University
Group Leader
-

Jingguang Chen
Thayer Lindsley Professor of Chemical Engineering, Columbia University
(631) 344-2655, jgchen@bnl.gov