- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Artificial Photosynthesis
Mechanistic insight into CO2 hydrogenation
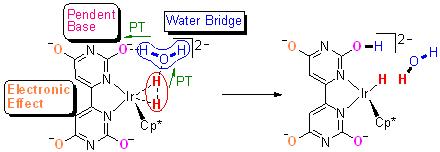 Reversible
H2 storage near room temperature and pressure with pH as the
‘switch’ for controlling the direction of the reaction has been demonstrated
using a bio-inspired proton-responsive Ir(III) dimer. Several similar
mononuclear Ir(III) catalysts for CO2 hydrogenation were prepared
to gain mechanistic insight through investigation of the factors that
control the effective generation of formate. These factors include: (1)
kinetic isotope effects by water, hydrogen, and bicarbonate; (2) position
and number of hydroxyl groups on bpy-type ligands; and (3) mono- vs di-nuclear
iridium complexes. We have, for the first time, obtained clear evidence from
kinetic isotope effects and computational studies of the involvement of a
water molecule in the rate-determining heterolysis of H2, and
accelerated proton transfer by formation of a water bridge in CO2
hydrogenation catalyzed by bio-inspired complexes bearing a pendent base. A
more significant enhancement of the catalytic activity was observed from
electron donation by the ligand than on the number of the active metal
centers.
Reversible
H2 storage near room temperature and pressure with pH as the
‘switch’ for controlling the direction of the reaction has been demonstrated
using a bio-inspired proton-responsive Ir(III) dimer. Several similar
mononuclear Ir(III) catalysts for CO2 hydrogenation were prepared
to gain mechanistic insight through investigation of the factors that
control the effective generation of formate. These factors include: (1)
kinetic isotope effects by water, hydrogen, and bicarbonate; (2) position
and number of hydroxyl groups on bpy-type ligands; and (3) mono- vs di-nuclear
iridium complexes. We have, for the first time, obtained clear evidence from
kinetic isotope effects and computational studies of the involvement of a
water molecule in the rate-determining heterolysis of H2, and
accelerated proton transfer by formation of a water bridge in CO2
hydrogenation catalyzed by bio-inspired complexes bearing a pendent base. A
more significant enhancement of the catalytic activity was observed from
electron donation by the ligand than on the number of the active metal
centers.
ACS Catal. 2013, 3, 856-860, DOI: 10.1021/cs400172j.




