- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Artificial Photosynthesis
Hydricity of Ruthenium(II) Hydride Complexes
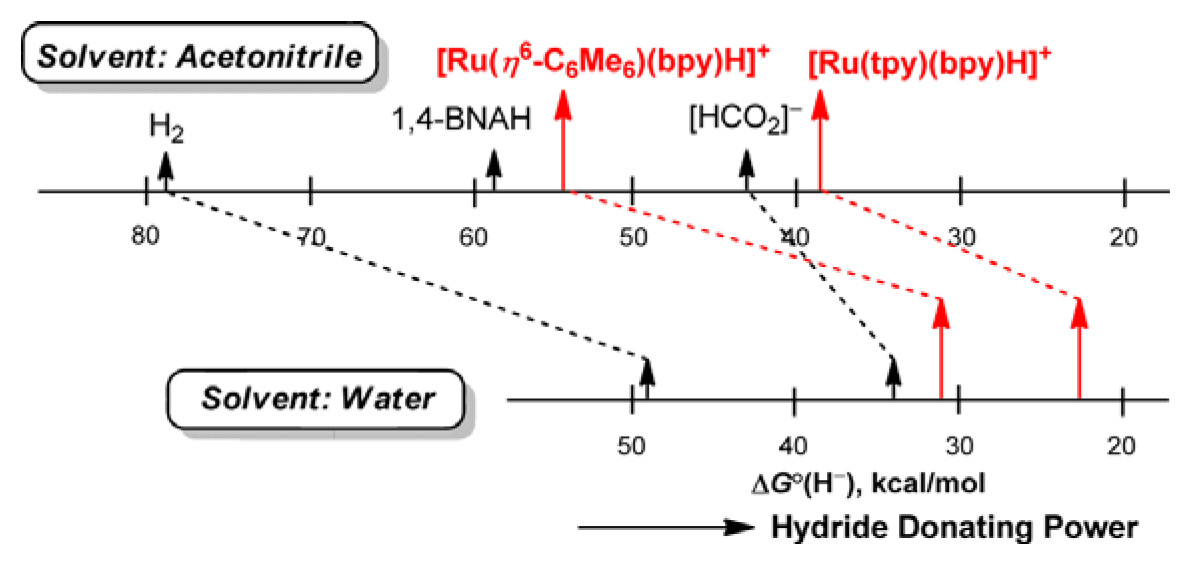 Despite
the fundamental importance of the hydricity of a transition metal
hydride (ΔGºH- for the reaction M–H
=> M+ + H–) in a range of reactions important in
catalysis and solar energy storage, ours (J. Am. Chem. Soc.
2009, 131, 2794) are the only values reported for water
solvent, and there has been no basis for comparison of these with the wider
range already determined for acetonitrile solvent, in particular. We have
used a variety of approaches to determine hydricity values in acetonitrile
of Ru(II) hydride complexes previously studied in water. Comparison of the
hydricity values for acetonitrile and water reveals a flattening or
compression of the hydricity range upon transferring the hydride complexes
to water.
Despite
the fundamental importance of the hydricity of a transition metal
hydride (ΔGºH- for the reaction M–H
=> M+ + H–) in a range of reactions important in
catalysis and solar energy storage, ours (J. Am. Chem. Soc.
2009, 131, 2794) are the only values reported for water
solvent, and there has been no basis for comparison of these with the wider
range already determined for acetonitrile solvent, in particular. We have
used a variety of approaches to determine hydricity values in acetonitrile
of Ru(II) hydride complexes previously studied in water. Comparison of the
hydricity values for acetonitrile and water reveals a flattening or
compression of the hydricity range upon transferring the hydride complexes
to water.
J. Am. Chem. Soc. 2012, 134, 15743-15757, DOI: 10.1021/ja302937q.




