- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Artificial Photosynthesis
Reversible Hydrogen Storage using CO2 and a Proton-Switchable Iridium Catalyst in Aqueous Media
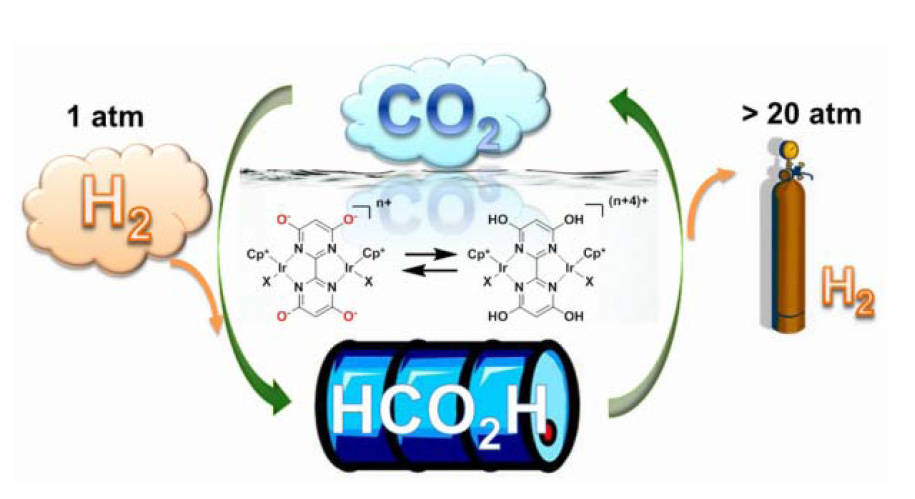 Green
plants convert carbon dioxide to sugar for energy storage via
photosynthesis. We investigated a novel catalyst that uses carbon dioxide
and hydrogen to store energy as formic acid. Using a homogeneous Ir catalyst
with a proton responsive ligand, we have achieved the first reversible and
recyclable hydrogen storage system that operates under mild conditions using
CO2, formate and formic acid. This system is energy-efficient and
green because it operates near ambient conditions, uses water as a solvent,
produces high-pressure CO-free hydrogen, and uses pH to control hydrogen
production or consumption. The extraordinary and switchable catalytic
activity is attributed to the multifunctional ligand which acts as a
proton-relay and strong π-donor, and is
rationalized by theoretical and experimental studies.
Green
plants convert carbon dioxide to sugar for energy storage via
photosynthesis. We investigated a novel catalyst that uses carbon dioxide
and hydrogen to store energy as formic acid. Using a homogeneous Ir catalyst
with a proton responsive ligand, we have achieved the first reversible and
recyclable hydrogen storage system that operates under mild conditions using
CO2, formate and formic acid. This system is energy-efficient and
green because it operates near ambient conditions, uses water as a solvent,
produces high-pressure CO-free hydrogen, and uses pH to control hydrogen
production or consumption. The extraordinary and switchable catalytic
activity is attributed to the multifunctional ligand which acts as a
proton-relay and strong π-donor, and is
rationalized by theoretical and experimental studies.
Nature Chemistry 2012, 4, 383-388, DOI: 10.1038/NCHEM.1295.




