- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Artificial Photosynthesis
Kinetics and Thermodynamics of Small Molecule Binding to Pincer-PCP Rhodium(I) Complexes
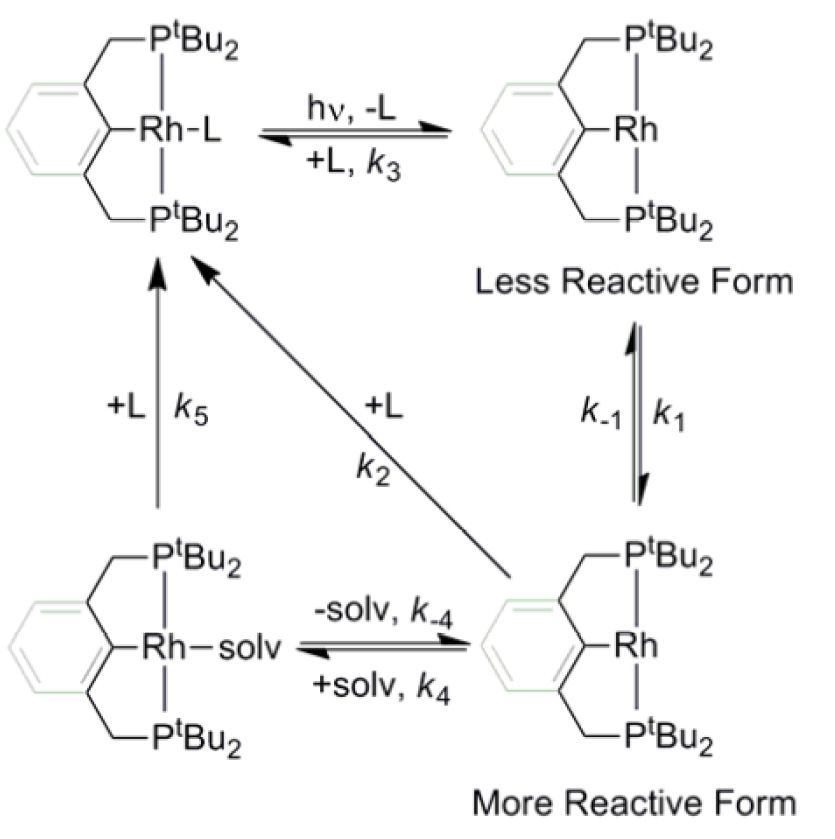 The kinetics and
thermodynamics of the binding of several small molecules (L
= N2, H2, D2, and C2H4)
to the coordinatively unsaturated pincer-PCP rhodium(I) complexes,
Rh[tBu2PCH2(C6H3)CH2PtBu2]
(1) and Rh[tBu2P(CH2)5PtBu2]
(2), in organic solvents (n-heptane, toluene, THF
and cyclohexane-d12) have been investigated by a
combination of kinetic flash photolysis methods, NMR equilibrium studies,
and density functional theory (DFT) calculations. Using various gas mixtures
and monitoring by NMR until equilibrium was established, the relative free
energies of binding of N2, H2 and C2H4
in cyclohexane-d12 were found to increase in the order,
C2H4 < N2 < H2. Time-resolved
infrared (TRIR) and UV-visible transient absorption spectroscopy revealed
that 355 nm excitation of 1–L and 2–L
results in the photoejection of ligand L. The subsequent mechanism of
binding of L to 1 and 2 to regenerate
1–L and 2–L is determined by the structure
of the PCP ligand framework and the nature of the solvent. In both cases,
the primary transient is a long-lived, unsolvated species. For 2,
we propose this to be an agostically-stabilized intermediate that is in
equilibrium with a more reactive, non-stabilized form, which reacts with L
at diffusion-controlled rates to regenerate 2–L. For
1, a similar mechanism is proposed to occur, but with an
additional parallel reaction pathway that involves the direct reaction of
the less-reactive form of 1 with L. Experiments in the more
coordinating solvent, THF revealed the binding of THF to 1
to generate 1–THF, and its subsequent reaction with L, as a
competing pathway.
The kinetics and
thermodynamics of the binding of several small molecules (L
= N2, H2, D2, and C2H4)
to the coordinatively unsaturated pincer-PCP rhodium(I) complexes,
Rh[tBu2PCH2(C6H3)CH2PtBu2]
(1) and Rh[tBu2P(CH2)5PtBu2]
(2), in organic solvents (n-heptane, toluene, THF
and cyclohexane-d12) have been investigated by a
combination of kinetic flash photolysis methods, NMR equilibrium studies,
and density functional theory (DFT) calculations. Using various gas mixtures
and monitoring by NMR until equilibrium was established, the relative free
energies of binding of N2, H2 and C2H4
in cyclohexane-d12 were found to increase in the order,
C2H4 < N2 < H2. Time-resolved
infrared (TRIR) and UV-visible transient absorption spectroscopy revealed
that 355 nm excitation of 1–L and 2–L
results in the photoejection of ligand L. The subsequent mechanism of
binding of L to 1 and 2 to regenerate
1–L and 2–L is determined by the structure
of the PCP ligand framework and the nature of the solvent. In both cases,
the primary transient is a long-lived, unsolvated species. For 2,
we propose this to be an agostically-stabilized intermediate that is in
equilibrium with a more reactive, non-stabilized form, which reacts with L
at diffusion-controlled rates to regenerate 2–L. For
1, a similar mechanism is proposed to occur, but with an
additional parallel reaction pathway that involves the direct reaction of
the less-reactive form of 1 with L. Experiments in the more
coordinating solvent, THF revealed the binding of THF to 1
to generate 1–THF, and its subsequent reaction with L, as a
competing pathway.
Inorg. Chem. 2013, 52, 4160-4172, DOI: 10.1021/ic300672g.




