- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Artificial Photosynthesis
Mechanism of the quenching of *[Ru(bpy)3]2+ by peroxodisulfate and its application for photoinduced oxidation reactions
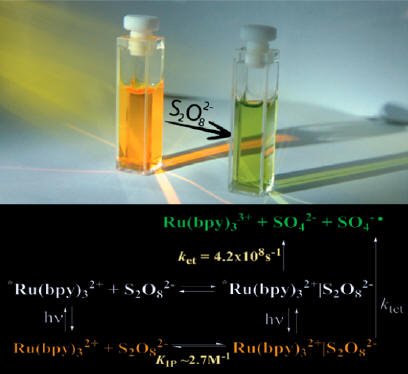 The earlier work
(J. Phys. Chem., 1984, 88, 1840) from Bard’s
laboratory has provided a model for the quenching of the excited state of
[RuII(bpy)3]2+ by
[S2O8]2- and was widely used since then
for the analysis of photo-driven oxidation reactions. In our work we resolve
several inconsistencies found for previously proposed model and explain the
mechanism of the oxidative quenching of the *[RuII(bpy)3]2+
by peroxydisulfate in terms of the mechanism consistent with the formation
of the precursor complex through the photo-excitation of the ground state
ion pair in addition to bimolecular quenching pathway. The proposed model
accurately describes experimental results for the quenching of *[Ru(bpy)3]2+
in a wide range of peroxydisulfate concentrations. The new model also
resolves some discrepancies in observed KIP and kET
compared to previously reported values based on the treatment proposed by
Bard et. al. It also provides convenient guidance for accurate evaluation of
photochemical parameters, such as quantum yields in photo-driven oxidation
reactions which employ [Ru(bpy)3]2+/persulfate couple.
The earlier work
(J. Phys. Chem., 1984, 88, 1840) from Bard’s
laboratory has provided a model for the quenching of the excited state of
[RuII(bpy)3]2+ by
[S2O8]2- and was widely used since then
for the analysis of photo-driven oxidation reactions. In our work we resolve
several inconsistencies found for previously proposed model and explain the
mechanism of the oxidative quenching of the *[RuII(bpy)3]2+
by peroxydisulfate in terms of the mechanism consistent with the formation
of the precursor complex through the photo-excitation of the ground state
ion pair in addition to bimolecular quenching pathway. The proposed model
accurately describes experimental results for the quenching of *[Ru(bpy)3]2+
in a wide range of peroxydisulfate concentrations. The new model also
resolves some discrepancies in observed KIP and kET
compared to previously reported values based on the treatment proposed by
Bard et. al. It also provides convenient guidance for accurate evaluation of
photochemical parameters, such as quantum yields in photo-driven oxidation
reactions which employ [Ru(bpy)3]2+/persulfate couple.
J. Phys Chem. A, 2013, 117, 10311–10319, DOI: 10.1021/jp407573d.




