- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Artificial Photosynthesis
Rapid Transfer of Hydride Ion from a Ruthenium Complex to C1 Species in Water
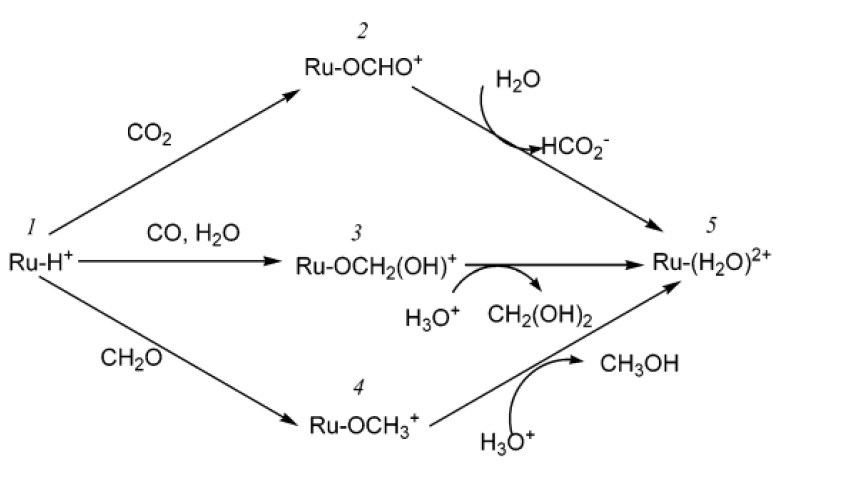 Water
is recognized as a desirable solvent for catalysis and as a promising raw
material for solar generation of fuels; however, relatively few kinetics and
mechanism studies of C1 reduction reactions in aqueous media have been
reported. We found that solvent water (Gutmann acceptor number, AN = 55)
accelerates the rate of reaction of [Ru(terpy)(bpy)H]+ with CO2
by more than 4 orders of magnitude compared to acetonitrile (AN = 18.9) (as
reported in Inorg. Chim. Acta 2000, 299,
155). Water also promotes the related reductions of carbon monoxide to
formaldehyde and of formaldehyde to methanol by the ruthenium(II) hydride
complex.
Water
is recognized as a desirable solvent for catalysis and as a promising raw
material for solar generation of fuels; however, relatively few kinetics and
mechanism studies of C1 reduction reactions in aqueous media have been
reported. We found that solvent water (Gutmann acceptor number, AN = 55)
accelerates the rate of reaction of [Ru(terpy)(bpy)H]+ with CO2
by more than 4 orders of magnitude compared to acetonitrile (AN = 18.9) (as
reported in Inorg. Chim. Acta 2000, 299,
155). Water also promotes the related reductions of carbon monoxide to
formaldehyde and of formaldehyde to methanol by the ruthenium(II) hydride
complex.
Inorg. Chem. 2010, 49, 9809-9822.




