- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Artificial Photosynthesis
Mechanistic understanding of low-energy pathways for catalytic water oxidation by Ru mononuclear polypyridyl complexes
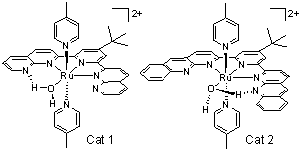 In
contrast to the proposed catalytic water oxidation mechanism by most of
single-site ruthenium complexes that proceed via the nucleophilic attack of
a water molecule on the RuV=O species, ruthenium(II) complexes containing
4-t-butyl-2,6-di-1',8'-(naphthyrid-2'-yl)-pyridine (or its bisbenzo-derivative),
water and two 4-picoline ligands show the reaction mechanism proceeding by
the thermodynamically more favorable “direct pathway” via [RuIV=O]2+, which
avoids the higher oxidation state [RuV=O]3+ in neutral and basic media. Our
experimental results on the pH-dependent onset catalytic potentials
indicative of a PCET driven low-energy pathway for the formation of products
with an O–O bond (such as [RuIII–OOH]2+ and [RuIV–OO]2+)
at an applied potential below the RuV=O/RuIV=O couple clearly support such a
mechanism. However, in the cases of [Ru(tpy)(bpy)(OH2)]2+
and [Ru(tpy)(bpm)(OH2)]2+, the formation of the RuV=O
species is required before O–O bond formation. Our complexes provide a
unique functional model for water oxidation that proceeds by four
consecutive PCET steps in neutral and alkaline media.
In
contrast to the proposed catalytic water oxidation mechanism by most of
single-site ruthenium complexes that proceed via the nucleophilic attack of
a water molecule on the RuV=O species, ruthenium(II) complexes containing
4-t-butyl-2,6-di-1',8'-(naphthyrid-2'-yl)-pyridine (or its bisbenzo-derivative),
water and two 4-picoline ligands show the reaction mechanism proceeding by
the thermodynamically more favorable “direct pathway” via [RuIV=O]2+, which
avoids the higher oxidation state [RuV=O]3+ in neutral and basic media. Our
experimental results on the pH-dependent onset catalytic potentials
indicative of a PCET driven low-energy pathway for the formation of products
with an O–O bond (such as [RuIII–OOH]2+ and [RuIV–OO]2+)
at an applied potential below the RuV=O/RuIV=O couple clearly support such a
mechanism. However, in the cases of [Ru(tpy)(bpy)(OH2)]2+
and [Ru(tpy)(bpm)(OH2)]2+, the formation of the RuV=O
species is required before O–O bond formation. Our complexes provide a
unique functional model for water oxidation that proceeds by four
consecutive PCET steps in neutral and alkaline media.
Inorg. Chem. 2013, 52, 8845–8850, DOI: 10.1021/ic401023w




