- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Artificial Photosynthesis
Water Oxidation by a Mononuclear Ruthenium Catalyst: Direct Pathway via Ru(IV)=O without forming Ru(V)=O
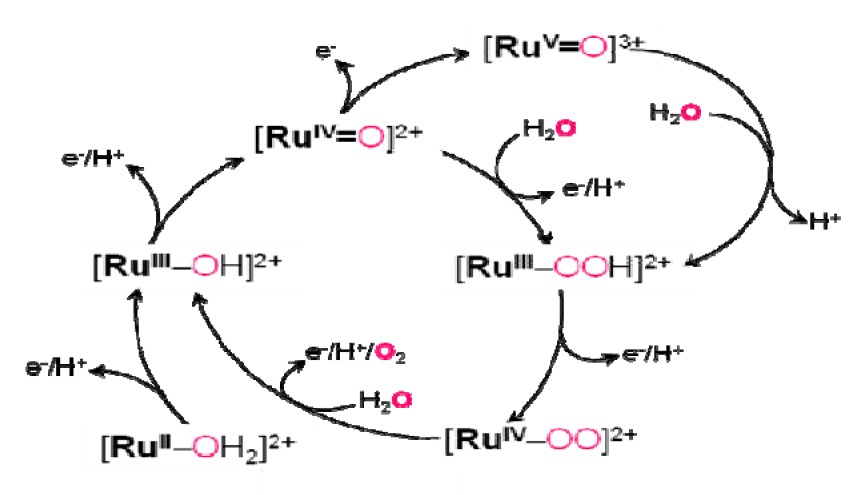 A detailed
characterization of intermediates in water oxidation catalyzed by
a mononuclear Ru polypyridyl complex [RuII–OH2]2+
(Ru = Ru complex with one 4-t-butyl-2,6-di-1',8'-(naphthyrid-2'-yl)-pyridine
ligand and two 4-picoline ligands) has been carried out using
electrochemistry, UV-vis and resonance Raman spectroscopy, pulse radiolysis,
stopped flow, and ESI-MS with H218O labeling
experiments, and theoretical calculations. The results reveal a number of
intriguing properties of intermediates such as [RuIV=O]2+
and [RuIV–OO]2+. At pH > 2.9, two consecutive
proton-coupled one-electron steps take place at the potential of the
[RuIII–OH]2+/[RuII–OH2]2+
couple, which is equal to or higher than the potential of the
[RuIV=O]2+/[RuIII–OH]2+
couple (i.e., the observation of a two-electron oxidation in cyclic
voltammetry). At pH 1, the rate constant of the first one-electron oxidation
by Ce(IV) is k1 = 2 × 104 M-1 s-1.
While pH-independent oxidation of [RuIV=O]2+ takes
place at 1420 mV vs NHE, bulk electrolysis of [RuII–OH2]2+
at 1260 mV vs NHE at pH 1 (0.1 M triflic acid) and 1150 mV at pH 6 (10 mM
sodium phosphate) yielded a red colored solution with a Coulomb count
corresponding to a net four-electron oxidation. ESI-MS with labeling
experiments clearly indicate that this species has an O–O bond. The
formation of [RuIII–OOH]2+ can proceed via formation
of [RuV=O]3+ followed by nucleophilic attack by a
water molecule at pH < 1, however this pathway cannot account for the
product formation at pH 6. A proposed alternative pathway is the reaction of
[RuIV=O]2+ with a water molecule accompanied by the
concomitant removal of an electron and a proton (“direct pathway”). The
direct pathway becomes predominant at higher pH in underpotential bulk
electrolysis experiments and in the onset of catalytic current in
background-subtracted CV scans as a function of pH.
A detailed
characterization of intermediates in water oxidation catalyzed by
a mononuclear Ru polypyridyl complex [RuII–OH2]2+
(Ru = Ru complex with one 4-t-butyl-2,6-di-1',8'-(naphthyrid-2'-yl)-pyridine
ligand and two 4-picoline ligands) has been carried out using
electrochemistry, UV-vis and resonance Raman spectroscopy, pulse radiolysis,
stopped flow, and ESI-MS with H218O labeling
experiments, and theoretical calculations. The results reveal a number of
intriguing properties of intermediates such as [RuIV=O]2+
and [RuIV–OO]2+. At pH > 2.9, two consecutive
proton-coupled one-electron steps take place at the potential of the
[RuIII–OH]2+/[RuII–OH2]2+
couple, which is equal to or higher than the potential of the
[RuIV=O]2+/[RuIII–OH]2+
couple (i.e., the observation of a two-electron oxidation in cyclic
voltammetry). At pH 1, the rate constant of the first one-electron oxidation
by Ce(IV) is k1 = 2 × 104 M-1 s-1.
While pH-independent oxidation of [RuIV=O]2+ takes
place at 1420 mV vs NHE, bulk electrolysis of [RuII–OH2]2+
at 1260 mV vs NHE at pH 1 (0.1 M triflic acid) and 1150 mV at pH 6 (10 mM
sodium phosphate) yielded a red colored solution with a Coulomb count
corresponding to a net four-electron oxidation. ESI-MS with labeling
experiments clearly indicate that this species has an O–O bond. The
formation of [RuIII–OOH]2+ can proceed via formation
of [RuV=O]3+ followed by nucleophilic attack by a
water molecule at pH < 1, however this pathway cannot account for the
product formation at pH 6. A proposed alternative pathway is the reaction of
[RuIV=O]2+ with a water molecule accompanied by the
concomitant removal of an electron and a proton (“direct pathway”). The
direct pathway becomes predominant at higher pH in underpotential bulk
electrolysis experiments and in the onset of catalytic current in
background-subtracted CV scans as a function of pH.
J. Am. Chem. Soc. 2011, 133, 14649-14665, DOI: 10.1021/ja203249e.




