- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Artificial Photosynthesis
Enabling light-driven water oxidation via a low-energy RuIV=O intermediate
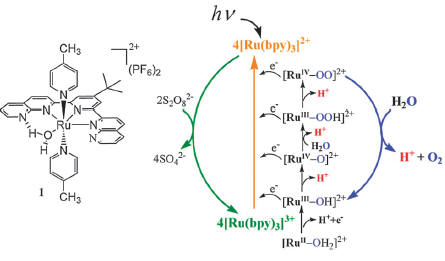 The discovery
of catalysts capable of driving water oxidation at relatively low
overpotential is a key challenge for efficient photoinduced water oxidation.
The mononuclear Ru(II) polypyridyl complex (1) has been
examined as a catalyst for visible-light-driven water oxidation in a
three-component homogeneous system containing [Ru(bpy)3]2+
as a photosensitizer, persulfate as a sacrificial electron acceptor and
1. We have successfully demonstrated that the earlier
proposed “direct pathway” takes place by the use of a mild oxidant such as
the photogenerated [Ru(bpy)3]3+ (1.26 V vs NHE) to
drive water oxidation at pH >3. The overall quantum yield of 9 % and a TOF
of 0.12 s-1 were found for photochemical water oxidation. These
values render 1 one of the most active mononuclear
ruthenium-based catalysts for light-driven water oxidation in a homogeneous
system.
The discovery
of catalysts capable of driving water oxidation at relatively low
overpotential is a key challenge for efficient photoinduced water oxidation.
The mononuclear Ru(II) polypyridyl complex (1) has been
examined as a catalyst for visible-light-driven water oxidation in a
three-component homogeneous system containing [Ru(bpy)3]2+
as a photosensitizer, persulfate as a sacrificial electron acceptor and
1. We have successfully demonstrated that the earlier
proposed “direct pathway” takes place by the use of a mild oxidant such as
the photogenerated [Ru(bpy)3]3+ (1.26 V vs NHE) to
drive water oxidation at pH >3. The overall quantum yield of 9 % and a TOF
of 0.12 s-1 were found for photochemical water oxidation. These
values render 1 one of the most active mononuclear
ruthenium-based catalysts for light-driven water oxidation in a homogeneous
system.
PCCP 2013, 15, 14058-14068, DOI: 10.1039/C3CP52038B.




