- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Artificial Photosynthesis
Steric Effect for Proton, Hydrogen-Atom, and Hydride Transfer Reactions with Geometric Isomers of NADH-Model Ruthenium Complexes
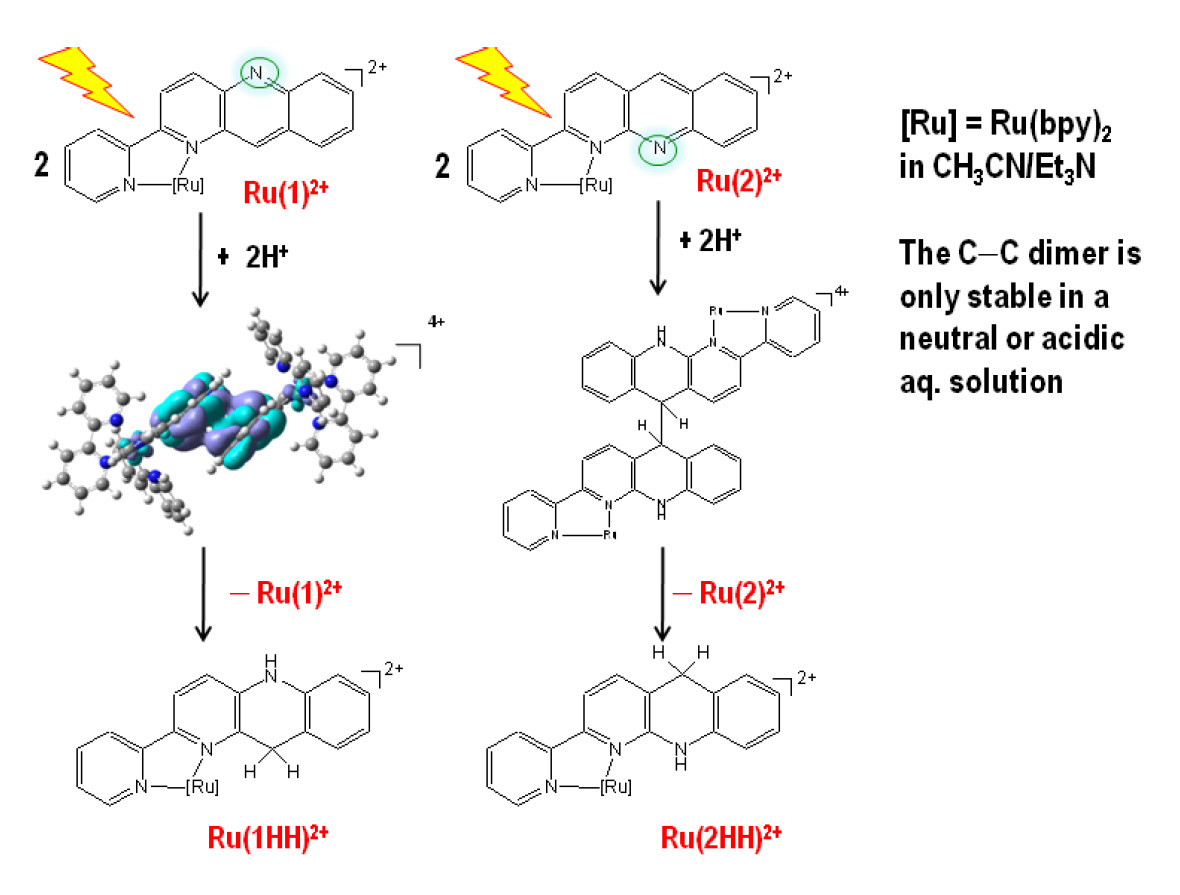 Two
isomers, [Ru(1)]2+ (Ru = Ru(bpy)2,
bpy = 2,2'-bipyridine, 1 = 2-(pyrid-2'-yl)-1-azaacridine)
and [Ru(2)]2+ (2 = 3-(pyrid-2'-yl)-4-azaacridine),
are bio-inspired model compounds containing the nicotinamide functionality
and can serve as precursors for the photogeneration of C–H hydrides for
studying reactions pertinent to the photochemical reduction of metal–C1
complexes and/or carbon dioxide. While it has been shown that the structural
differences between the azaacridine ligands of [Ru(1)]2+
and [Ru(2)]2+ have a significant effect on the
mechanism of formation of the hydride donors, [Ru(1HH)]2+
and [Ru(2HH)]2+, in aqueous solution, we
describe the steric implications for proton, net-hydrogen-atom and
net-hydride transfer reactions in this work. Protonation of [Ru(2•–)]+
in aprotic and even protic media is slow compared to that of [Ru(1•–)]+.
The net hydrogen-atom transfer between *[Ru(1)]2+
and H2Q proceeds by one-step electron-proton transfer (EPT),
rather than sequential EPT. Such a reaction was not observed for *[Ru(2)]2+
because the non-coordinated N atom is not readily available for an
interaction with H2Q. Finally, the rate of the net hydride ion
transfer from [Ru(1HH)]2+ to [Ph3C]+
is significantly slower than that of [Ru(2HH)]2+
owing to steric congestion at the donor site.
Two
isomers, [Ru(1)]2+ (Ru = Ru(bpy)2,
bpy = 2,2'-bipyridine, 1 = 2-(pyrid-2'-yl)-1-azaacridine)
and [Ru(2)]2+ (2 = 3-(pyrid-2'-yl)-4-azaacridine),
are bio-inspired model compounds containing the nicotinamide functionality
and can serve as precursors for the photogeneration of C–H hydrides for
studying reactions pertinent to the photochemical reduction of metal–C1
complexes and/or carbon dioxide. While it has been shown that the structural
differences between the azaacridine ligands of [Ru(1)]2+
and [Ru(2)]2+ have a significant effect on the
mechanism of formation of the hydride donors, [Ru(1HH)]2+
and [Ru(2HH)]2+, in aqueous solution, we
describe the steric implications for proton, net-hydrogen-atom and
net-hydride transfer reactions in this work. Protonation of [Ru(2•–)]+
in aprotic and even protic media is slow compared to that of [Ru(1•–)]+.
The net hydrogen-atom transfer between *[Ru(1)]2+
and H2Q proceeds by one-step electron-proton transfer (EPT),
rather than sequential EPT. Such a reaction was not observed for *[Ru(2)]2+
because the non-coordinated N atom is not readily available for an
interaction with H2Q. Finally, the rate of the net hydride ion
transfer from [Ru(1HH)]2+ to [Ph3C]+
is significantly slower than that of [Ru(2HH)]2+
owing to steric congestion at the donor site.
Faraday Discuss. 2012, 155, 129-144, DOI: 10.1039/c1fd00094b.




