Direct View of Tantalum Oxidation that Impedes Qubit Coherence
Advanced electron microscopy and computational modeling reveal clues that may help improve performance of superconducting components for quantum computers
February 5, 2024
 enlarge
enlarge
Junsik Mun, a research associate in the Center for Functional Nanomaterials (CFN) and Condensed Matter Physics & Materials Science (CMPMS) department at Brookhaven National Laboratory and first author on the study, at the scanning transmission electron microscope in CMPMS. This instrument was used to detect details of tantalum, a superconductor with promising properties for making qubits. (Jessica Rotkiewicz/Brookhaven National Laboratory)
UPTON, NY—Scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory and DOE’s Pacific Northwest National Laboratory (PNNL) have used a combination of scanning transmission electron microscopy (STEM) and computational modeling to get a closer look and deeper understanding of tantalum oxide. When this amorphous oxide layer forms on the surface of tantalum—a superconductor that shows great promise for making the “qubit” building blocks of a quantum computer—it can impede the material’s ability to retain quantum information. Learning how the oxide forms may offer clues as to why this happens—and potentially point to ways to prevent quantum coherence loss. The research was recently published in the journal ACS Nano.
The paper builds on earlier research by a team at Brookhaven’s Center for Functional Nanomaterials (CFN), Brookhaven’s National Synchrotron Light Source II (NSLS-II), and Princeton University that was conducted as part of the Co-design Center for Quantum Advantage (C2QA), a Brookhaven-led national quantum information science research center in which Princeton is a key partner.
“In that work, we used X-ray photoemission spectroscopy at NSLS-II to infer details about the type of oxide that forms on the surface of tantalum when it is exposed to oxygen in the air,” said Mingzhao Liu, a CFN scientist and one of the lead authors on the study. “But we wanted to understand more about the chemistry of this very thin layer of oxide by making direct measurements,” he explained.
 enlarge
enlarge
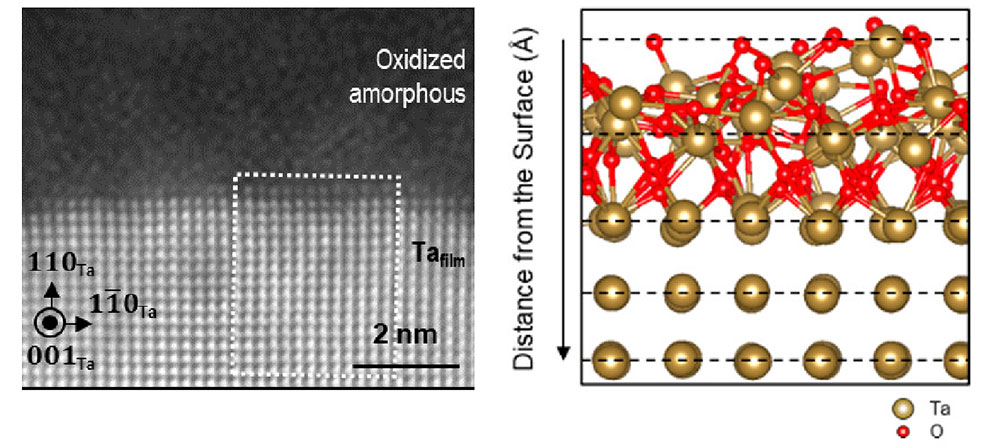
Left: This scanning transmission electron microscope (STEM) image of a tantalum (Ta) film surface shows an amorphous oxide above the regularly arrayed atoms of crystalline Ta metal. Right: The STEM imaging combined with computational modeling revealed details of the interface between these layers, including the formation of the amorphous oxide (top layer) and a suboxide layer that retains crystalline features (second layer) above the regularly arrayed tantalum atoms. (Brookhaven National Laboratory)
So, in the new study, the team partnered with scientists in Brookhaven’s Condensed Matter Physics & Materials Science (CMPMS) Department to use advanced STEM techniques that enabled them to study the ultrathin oxide layer directly. They also worked with theorists at PNNL who performed computational modeling that revealed the most likely arrangements and interactions of atoms in the material as they underwent oxidation. Together, these methods helped the team build an atomic-level understanding of the ordered crystalline lattice of tantalum metal, the amorphous oxide that forms on its surface, and intriguing new details about the interface between these layers.
“The key is to understand the interface between the surface oxide layer and the tantalum film because this interface can profoundly impact qubit performance,” said study co-author Yimei Zhu, a physicist from CMPMS, echoing the wisdom of Nobel laureate Herbert Kroemer, who famously asserted, “The interface is the device.”
Emphasizing that “quantitatively probing a mere one-to-two-atomic-layer-thick interface poses a formidable challenge,” Zhu noted, “we were able to directly measure the atomic structures and bonding states of the oxide layer and tantalum film as well as identify those of the interface using the advanced electron microscopy techniques developed at Brookhaven.”
 enlarge
enlarge
Members of the research team that uncovered details of tantalum oxide and suboxide in front of the scanning transmission electron microscope in the Condensed Matter Physics & Materials Science Department at Brookhaven National Laboratory, left to right: Xiaohui Qu (CFN), Junsik Mun (CFN & CMPMSD), Yimei Zhu (CMPMS), Mingzhao Liu (CFN), Chenyu Zhou (CFN). (Jessica Rotkiewicz/Brookhaven National Laboratory)
“The measurements reveal that the interface consists of a ‘suboxide’ layer nestled between the periodically ordered tantalum atoms and the fully disordered amorphous tantalum oxide. Within this suboxide layer, only a few oxygen atoms are integrated into the tantalum crystal lattice,” Zhu said.
The combined structural and chemical measurements offer a crucially detailed perspective on the material. Density functional theory calculations then helped the scientists validate and gain deeper insight into these observations.
“We simulated the effect of gradual surface oxidation by gradually increasing the number of oxygen species at the surface and in the subsurface region,” said Peter Sushko, one of the PNNL theorists.
By assessing the thermodynamic stability, structure, and electronic property changes of the tantalum films during oxidation, the scientists concluded that while the fully oxidized amorphous layer acts as an insulator, the suboxide layer retains features of a metal.
“We always thought if the tantalum is oxidized, it becomes completely amorphous, with no crystalline order at all,” said Liu. “But in the suboxide layer, the tantalum sites are still quite ordered.”
 enlarge
enlarge
Peter Sushko, a theorist at DOE's Pacific Northwest National Laboratory involved in the computational modeling that helped explain the properties of tantalum during oxidation. (PNNL)
With the presence of both fully oxidized tantalum and a suboxide layer, the scientists wanted to understand which part is most responsible the loss of coherence in qubits made of this superconducting material.
“It’s likely the oxide has multiple roles,” Liu said.
First, he noted, the fully oxidized amorphous layer contains many lattice defects. That is, the locations of the atoms are not well defined. Some atoms can shift around to different configurations, each with a different energy level. Though these shifts are small, each one consumes a tiny bit of electrical energy, which contributes to loss of energy from the qubit.
“This so-called two-level system loss in an amorphous material brings parasitic and irreversible loss to the quantum coherence—the ability of the material to hold onto quantum information,” Liu said.
But because the suboxide layer is still crystalline, “it may not be as bad as people were thinking,” Liu said. Maybe the more-fixed atomic arrangements in this layer will minimize two-level system loss.
Then again, he noted, because the suboxide layer has some metallic characteristics, it could cause other problems.
“When you put a normal metal next to a superconductor, that could contribute to breaking up the pairs of electrons that move through the material with no resistance,” he noted. “If the pair breaks into two electrons again, then you will have loss of superconductivity and coherence. And that is not what you want.”
Future studies may reveal more details and strategies for preventing loss of superconductivity and quantum coherence in tantalum.
This research was funded by the DOE Office of Science. In addition to the experimental facilities described above, this research used computational resources at CFN and at the National Energy Research Scientific Computing Center (NERSC) at DOE’s Lawrence Berkeley National Laboratory. CFN, NSLS-II, and NERSC are DOE Office of Science user facilities.
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Follow @BrookhavenLab on social media. Find us on Instagram, LinkedIn, X, and Facebook.
2024-21676 | INT/EXT | Newsroom