Inhibitor or Activator: Why not Both?
Scientists discovered how a protein that plays an important role in various diseases multitasks
January 31, 2020
 enlarge
enlarge
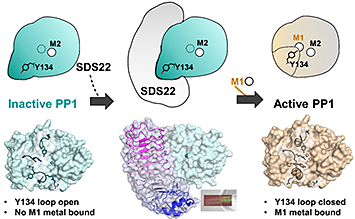
To activate PP1, SDS22 needs to bind to and stabilize it. Then, through an unknown mechanism, a zinc ion (Zn2+) is loaded into PP1's metal site, M1. Only then can SDS22 release from the now active PP1. Image credit: PNAS 116 (41) 20472-20481 (2019)]
The Science
Scientists discovered how the regulatory protein SDS22 acts as both an inhibitor and an activator for protein phosphatase 1 (PP1).
The Impact
PP1, with PP2A, accounts for more than 90% of the phosphatase activity in eukaryotes. Understanding how PP1 is activated will lead to new treatments for PP1-related diseases, such as cancer.
Summary
PP1, a metal-dependent enzyme responsible for more than 50% of all dephosphorylation reactions, forms distinct holoenzymes with more than 200 regulatory proteins, which create specificity for its substrates. Therefore, understanding how PP1 is activated will lead to new treatments for PP1-related diseases, such as cancer.
Intriguingly, one of its most ancient regulators, SDS22, is not only an essential gene but also is both a PP1 inhibitor and an activator. How such divergent functions are mediated by the same regulator is, so far, unknown.
By using biochemical, x-ray crystallographic, and cellular approaches, researchers determined that SDS22 binds a unique, metal-deficient conformation of PP1 that renders PP1 inactive. Furthermore, once the complex forms, it does not permanently dissociate until PP1 binds a M1 metal. The scientists used the Frontier Microfocus Macromolecular Crystallography (FMX) beamline to measure the crystallized protein structure and identity the native metals. The FMX is beamline is part of the life science capability suite at the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy Office of Science User Facility located at Brookhaven National Laboratory.
Their results provide a new framework for understanding how the PP1 holoenzyme assembly is regulated; namely, that SDS22 is a "PP1 trap," providing a pool of PP1 poised for the rapid formation of new holoenzymes in response to dynamically changing cellular events. Understanding the mechanisms of PP1 holoenzyme assembly and activation will lead to novel therapies for cancer, drug addiction, and the scores of other diseases associated with PP1 signaling.
Download the research summary slide
Contact
Kelly Tatchell
Louisiana State University Health Sciences Center
ktatch@lsuhsc.edu
Rebecca Page
University of Arizona
rebeccapage@email.arizona.edu
Publications
M. S. Choy, T. M. Moon, R. Ravindran, J. A. Bray, L. C. Robinson, T. L. Archuleta, W. Shi, W. Peti, K. Tatchell, R. Page. “SDS22 selectively recognizes and traps metal-deficient inactive PP1”. PNAS 116 (41) 20472-20481 (2019). DIO: https://doi.org/10.1073/pnas.1908718116
Funding
This work was supported by Grant R01GM134683 from the National Institute of General Medicine to W.P., and Grant R01GM098482 from the National Institute of General Medicine to R.P. Portions of this work were also supported by the Department of Biochemistry and Molecular Biology, the Feist-Weiller Cancer Center, and the Research Core facility at Louisiana State University Health Sciences Center (LSUHSC)-Shreveport. R.R. was supported by an Ike Muslow Predoctoral Fellowship awarded by the School of Graduate Studies, LSUHSC-Shreveport. Work at the FMX (Frontier Microfocus Macromolecular Crystallography; 17-ID-2) beamline was supported by the National Institute of Health, National Institute of General Medical Sciences (Grant P41GM111244), and by the DOE Office of Biological and Environmental Research (Grant KP1605010). The National Synchrotron Light Source II at Brookhaven National Laboratory was supported by the DOE Office of Basic Energy Sciences under Contract DE-SC0012704 (KC0401040). This research also used beamline 12-2 at the Stanford Synchrotron Radiation Lightsource. Use of the Stanford Synchrotron Radiation Lightsource (SSRL), Stanford Linear Accelerator Center (SLAC) is supported by the US Department of Energy, Office of Science and Office of Basic Energy Sciences under Contract DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program was supported by the DOE Office of Biological and Environmental Research and by the National Institutes of Health, National Institute of General Medical Sciences (including Grant P41GM103393).
2020-17164 | INT/EXT | Newsroom









