Fighting Cancer with a New Antibody
Mutant Gene-Targeted approach offers potential to broadly expand the benefit of immunotherapies
June 30, 2021
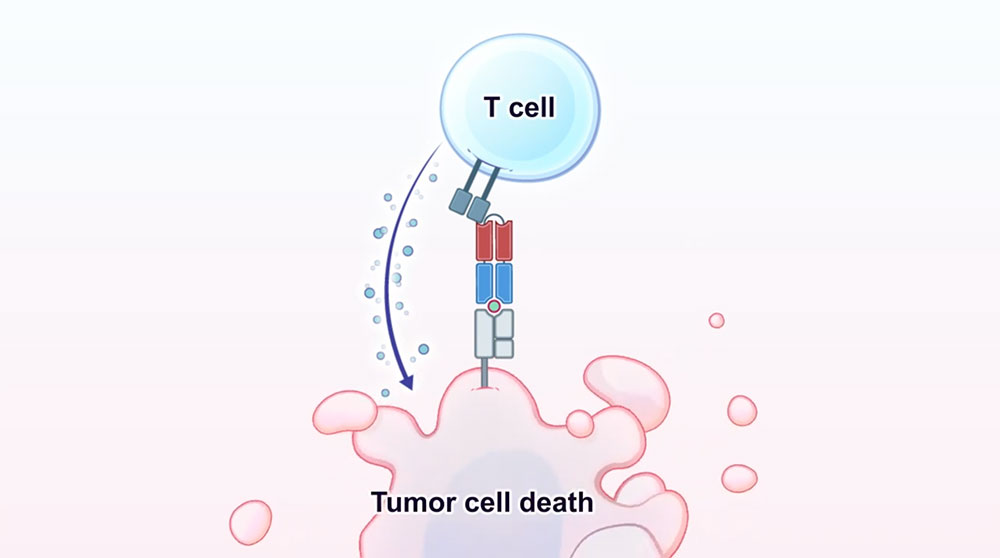
This illustration shows how the T-cell attacks the tumor cell after it was activated by the antibody. Image credit: Science, 371, 6533 (2021)
The Science
Scientists identified a highly specific antibody to combat cancer cells in tumors with the most common genetic mutation, TP53.
The Impact
TP53 is the most common cancer-driver gene, however, drugs that target mutant tumor suppressor genes are not available. This study shows the first results of an immuno-therapeutic agent that targets TP53, effectively killing tumor cells in vitro and suppressing tumor growth in mice.
Summary
Targeted therapies, such as trastuzumab and imatinib, have revolutionized the treatment of cancer patients. These drugs generally inhibit the action of proteins instead of reactivating the existing tumor suppressor genes. TP53 (tumor protein P53), a tumor suppressor gene, is the most commonly mutated cancer-driver gene. Nevertheless, drugs that target mutant p53, the protein product, remain unavailable currently, decades after the discovery of its critical role in cancer.
In this study, a team of researchers developed an antibody-based therapeutic approach that targets a neoantigen, derived from a common TP53 mutation, in a highly specific fashion. It effectively activates T-cells and kills tumor cells, both in vitro and in vivo, despite the low antigen density on their surface.
Among other research techniques, the team collected data at the Highly Automated Macromolecular Crystallography (AMX) and Frontier Microfocusing Macromolecular Crystallography (FMX) beamlines to analyze the atomic structure of the antibody. AMX and FMX are part of the comprehensive suite of beamlines for structural biology at the National Synchrotron Light Source II (NSLS-II). NSLS-II is a U.S. Department of Energy Office of Science user facility located at DOE’s Brookhaven National Laboratory.
This research represents an essential first step in the long journey to developing an “off-the-shelf- therapeutic” based on the new antibody for successfully treating cancer patients. In addition, this approach could, in theory, be used to treat cancers containing other mutations that are difficult to target by conventional means.
Download the research summary slide
Related Links
Press Release: Mutant Gene-Targeted Immunotherapy Approach Developed
Contact
Bert Vogelstein
John Hopkins Medicine
vogelbe@jhmi.edu
Sandra B. Gabelli
John Hopkins Medicine
gabelli@jhmi.edu
Publications
E. Han-Chung Hsiue, K. M. Wright, J. Douglass, M. S. Hwang, B. J. Mog, A. H. Pearlman, S. Paul, S. R. DiNapoli, M. F. Konig, Q. Wang, A. Schaefer, M. S. Miller, A. D. Skora, P. A. Azurmendi, M. B. Murphy, Q. Liu, E. Watson, Y. Li, D. M. Pardoll, C. Bettegowda, N. Papadopoulos, K. W. Kinzler, B. Vogelstein, S. B. Gabelli, S. Zhou, Targeting a neoantigen derived from a common TP53 mutation, Science, 371, 6533 (2021), DOI: 10.1126/science.abc8697
Funding
This work was supported by the Virginia and D. K. Ludwig Fund for Cancer Research, the Lustgarten Foundation for Pancreatic Cancer Research, the Commonwealth Fund, the Bloomberg~Kimmel Institute for Cancer Immunotherapy, Bloomberg Philanthropies, the Mark Foundation for Cancer Research, and NIH Cancer Center support grant P30 CA006973. J.D., B.J.M., A.H.P., and S.R.D. were supported by NIH grant T32 GM73009. S.P. was supported by NIH grant 5T32 CA009071-38 and SITC-Amgen Cancer Immunotherapy in Hematologic Malignancies Fellowship. M.F.K. was supported by NIH grant T32 AR048522. C.B. was supported by NCI grant R37 CA230400. Work at the AMX (17-ID-1) and FMX (17-ID-2) beamlines was supported by the NIH, the National Institute of General Medical Sciences (P41GM111244), the U.S. Department of Energy (DOE) Office of Biological and Environmental Research (KP1605010), and the National Synchrotron Light Source II at Brookhaven National Laboratory, which is supported by the DOE Office of Basic Energy Sciences under contract DE-SC0012704 (KC0401040).
2021-19014 | INT/EXT | Newsroom