X-rays Reveal Elusive Chemistry for Better EV Batteries
Scientists gain new insights on stability issues of lithium metal anodes
February 8, 2023
 enlarge
enlarge
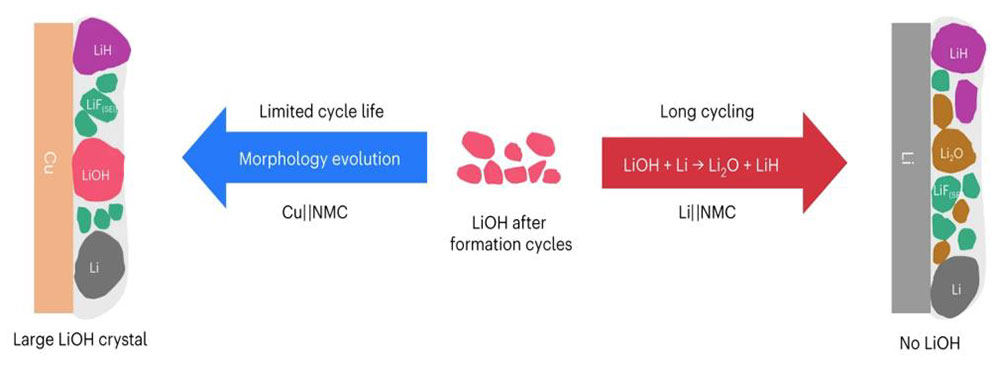
Schematic picture of how the solid-electrolyte interphase component evolves during battery cycling based on X-ray diffraction results. Image credit: S. Tan, et. al. Nat. Nanotechnol. (2022).
The Science
High energy x-rays enabled scientists to probe the solid-electrolyte interphase, a sensitive chemical layer in batteries that's key to stabilizing lithium metal anodes.
The Impact
By stabilizing the lithium metal anode, these batteries have the potential to provide more than double the energy density as traditional graphite anodes.
Summary
Lithium-ion batteries have become common due to their high efficiency and long life, but these batteries face challenges in more demanding applications, such as powering electric vehicles over long distances. Lithium metal anodes offer a much higher energy density than more traditional graphite anodes. But one of the biggest challenges scientists currently face is finding a way to stabilize the anode as the battery charges and discharges. The key to this stabilization is controlling the chemistry of a chemical layer in batteries called the solid-electrolyte interphase (SEI).
The sensitivity of the SEI makes it very challenging to study. But the high-energy x-rays at the X-ray Powder Diffraction (XPD) beamline at the National Synchrotron Light Source II (NSLS-II) provide less radiation damage to the sample. And by combining synchrotron-based X-ray diffraction and pair distribution function analysis, the scientists revealed a much more convoluted formation mechanism of SEI, with significant contributions from electrolyte, cathode, moisture, and native surface species on lithium metal, and with a highly dynamic nature during cycling.
Using isotope labeling of the solvent, the team traced the origin of LiH to electrolyte solvent, moisture, and a new source: the native surface species (LiOH) on pristine Li metal. When lithium accessibility is very limited as in the case of anode-free cells, LiOH develops into plate-shaped large crystals during cycling. Alternatively, if the lithium source is abundant, LiOH reacts with Li metal to form LiH and Li2O. While the desired anion-derived LiF-rich SEI is typically found in the concentrated electrolytes or their derivatives, the team found that it can also be formed in a low-concentration electrolyte via the crosstalk effect.
Elucidation of the formation and cycling pathways of these components revealed opportunities for low-cost electrolyte development.
Download the research summary slide (PDF)
Related Links
Press Release: “X-rays Reveal Elusive Chemistry for Better EV Batteries”
Contact
Enyuan Hu
Chemistry Division, Brookhaven National Laboratory
enhu@bnl.gov
Publication
S. Tan, J.M. Kim, A. Corrao, S. Ghose, H. Zhong, N. Rui, X. Wang, S. Senanayake, B.J. Polzin, P. Khalifah, J. Xiao, J. Liu, K. Xu, X.-Q. Yang, X. Cao, E. Hu. Unravelling the convoluted and dynamic interphasial mechanisms on Li metal anodes. Nat. Nanotechnol. (2022). DOI: 10.1038/s41565-022-01273-3
Funding
S.T., A.C., P.K., X.-Q.Y., and E.H. at BNL are supported by the Assistant Secretary for Energy Efficiency and Renewable Energy (EERE), Vehicle Technology Office (VTO) of the US Department of Energy (DOE) through the Advanced Battery Materials Research (BMR) Program, including Battery500 Consortium under contract no. DE-SC0012704. This research used 28-ID-2 and 7-BM beamlines of the National Synchrotron Light Source II, US DOE Office of Science User Facilities operated for the DOE Office of Science by BNL under contract no. DE-SC0012704. DFT computational work used the resources of the Center for Functional Nanomaterials, a US DOE Office of Science User Facility at BNL, under contract no. DE-SC0012704. J-M.K., J.X., J.L. and X.C. at Pacific Northwest National Laboratory (PNNL) also thank support from EERE and VTO of the US DOE through the BMR program including Battery500 Consortium. The XPS were conducted in the William R. Wiley Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by DOE’s Office of Biological and Environmental Research and located at PNNL. PNNL is operated by Battelle for US DOE under contract DE-AC05-76RL01830. The electrodes used in this study were produced at the US DOE’s CAMP (Cell Analysis, Modelling and Prototyping) Facility, Argonne National Laboratory. The CAMP Facility is fully supported by VTO, EERE of US DOE. K.X. thanks the financial aid from Joint Center of Energy Storage Research, an Energy Hub funded by US DOE, Office of Basic Energy Science.
2023-21156 | INT/EXT | Newsroom









