How Water Salinity Affects Contaminants in Coastal Urban Soil
March 11, 2024
 enlarge
enlarge
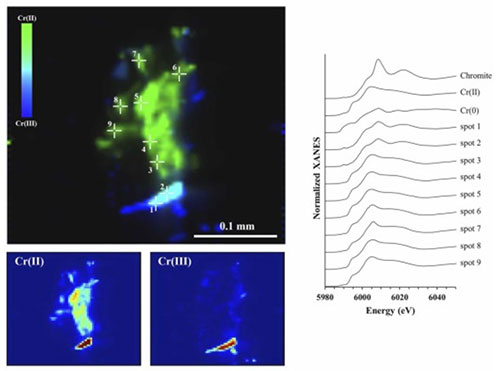
Oxidation state maps of a contaminated soil sample. The green and blue colors indicate Cr(II), and chromite [Cr(III)] hotspots, respectively. The crosses represent specific locations where μ-XANES spectra were acquired. Credit: Journal of Hazard. Mater. 462: 132661 (2024)
The Science
Seawater and low oxygen conditions resulted in lower release of the contaminant Chromium (Cr) from soil compared to freshwater and aerobic conditions which significantly increased the release.
The Impact
The toxicity of Cr is dependent on its oxidation state. Learning how environmental factors, like water salinity and oxygen content, change that state could have ecological impact.
Summary
As sea levels rise, it’s important to note how the chemical composition of the surrounding water interacts with the soil it comes into contact with. Chromium, a toxic element that is sensitive to changes in oxidation states, has been found in some contaminated coastal soils. The toxicity and carcinogenicity of chromium is dependent on its oxidation state or "species," so understanding these chemical reactions could make an impact in these environments. Researchers wanted to understand how the water salinity and oxygen levels affected the species of chromium found in and released from the soil in coastal cities.
Research led by a team from the University of Delaware characterized the chromium found in soil samples from the 12th Street chemical drum site in Wilmington, Delaware. The team examined the samples with microfocused x-ray fluorescence microscopy and spectroscopy at the X-ray Fluorescence Microscopy (XFM) beamline at the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy Office of Science User Facility at Brookhaven National Laboratory. Unique features in the spectra were used to identify the samples and identify their composition. As a result of these measurements paired with other synchrotron-based x-ray spectroscopy studies and wet chemical analyses, the scientists found the soils to contain very high levels of chromium and determined that chromite and iron-chromium hydroxide were the predominant species. It was found that saltwater with low oxygen conditions released less chromium from the soil compared to freshwater with aerobic conditions— which released 3 – 8 times more chromium! The chemical reactions between the minerals in the water and the chromium in the soil, a reaction that changes oxidation in a material known as redox, played a significant role in this difference. Understanding more about this process could play a role in the health of our coastal environments.
Download the research summary slide (PDF)
Contact
Piyapas Sricharoenvech
University of Delaware
pypschrv@udel.edu
Publications
P Sricharoenvech, MG Siebecker, R Tappero, G Landrot, MHH Fischel, DL Sparks. Chromium speciation and mobility in contaminated coastal urban soils affected by water salinity and redox conditions. J Hazard Mater. 462:132661 (2024). doi: 10.1016/j.jhazmat.2023.132661
Funding
The authors thank the TracE Analytical Metals (TEAM) Lab at Johns Hopkins Bloomberg School of Public Health for HF digestion of soil samples, and Dr. Steve Sutton at Advanced Photon Source, Argonne National Laboratory, for providing the Cr(II) glass standard. We also thank the staff in the Soil Testing and the Advanced Materials Characterization Labs at the University of Delaware for soil characterization and ICP analyses. P.S. thanks the Anandamahidol foundation, Thailand, for providing her doctoral fellowship at the University of Delaware. Support is also acknowledged from USDA multi-state project NC1187. This research used beamline 4-BM (XFM) of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. Lastly, the authors acknowledge SOLEIL for provision of synchrotron radiation facilities.
2024-21784 | INT/EXT | Newsroom









