Deciphering Oxide Reduction at the Atomic Scale
October 16, 2025
 enlarge
enlarge
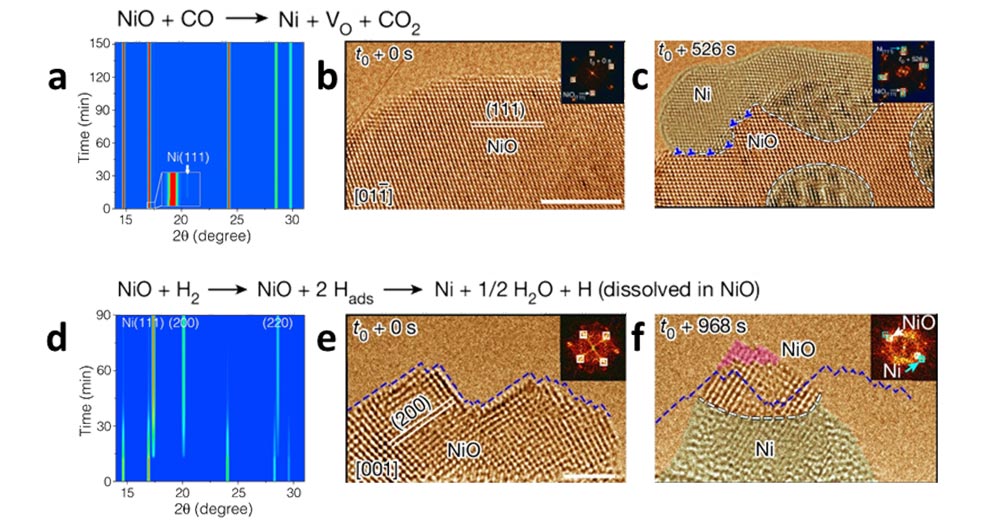
(a) In-situ synchrotron X-ray diffraction (XRD) measurements of NiO reduction under CO flow. A weak Ni(111) reflection appears early (inset), indicating rapid nucleation of metallic Ni, but its intensity remains low over time — suggesting self-limiting surface reduction. (b, c) Time-resolved HRTEM images showing NiO reduction at 400°C under CO pressures of approximately 1 Pa. (d) Time-resolved XRD during NiO reduction under H2 shows an initial incubation period, followed by rapid and sustained growth of Ni(111), (200), and (220) reflections, consistent with bulk phase transformation to metallic Ni. (e, f) In situ HRTEM images showing NiO reduction at 400°C under H2 pressures of approximately approximately 0.08 Pa.
The Science
Scientists reveal that CO and H2 reduce metal oxides through fundamentally different atomic pathways that were once thought to be similar.
The Impact
This work paves the way for improved catalyst design and more efficient metal production.
Summary
Carbon monoxide (CO) and hydrogen (H2) are common reductants used in catalysis, metal production, and energy technologies. It was often thought that these reactions followed similar pathways, but a collaborative team of scientists from Binghamton University, Stony Brook University, Columbia University, and Brookhaven National Laboratory have found, under careful observation, that this is not the case.
The researchers directly watched the reduction of nickel oxide (NiO) into metallic nickel in real time using environmental transmission electron microscopy (TEM). This enabled them to image atoms and capture video while gases flow at high temperature. They found that H2 and CO don’t reduce metal oxides the same way. In NiO, CO strips oxygen only at the surface and quickly builds a thin nickel layer that blocks further reaction. Hydrogen sends tiny protons into the crystal, helping oxygen vacancies move inward so the whole particle converts to metal from the inside out.
To validate their findings, the team took their research to the Quick X-ray Absorption and Scattering beamline at the National Synchrotron Light Source II, a user facility at the Department of Energy’s Brookhaven National Laboratory. There, they performed in-situ X-ray diffraction. At the Center for Functional Nanomaterials, another user facility at Brookhaven National Laboratory, they performed near-ambient-pressure X-ray photoelectron spectroscopy and a residual gas analysis to monitor NiO reduction on a larger scale. They then used computer modeling (DFT) to explain the atom-level steps.
This atomic scale understanding of the reduction process could be helpful in guiding metallurgical processes and improved catalyst design. The choice of gas can significantly influence where and how metal forms, directly impacting a catalyst’s activity, durability, and selectivity.
Download the research summary slide (PDF)
Related Links
Paper: Atomic dynamics of gas-dependent oxide reducibility
Contact
Guangwen Zhou
State University of New York at Binghamton
gzhou@binghamton.edu
Publications
Xiaobo Chen, Jianyu Wang, Shyam Bharatkumar Patel, Shuonan Ye, Yupeng Wu, Zhikang Zhou, Linna Qiao, Yuxi Wang, Nebojsa Marinkovic, Meng Li, Sooyeon Hwang, Dmitri N. Zakharov, Lu Ma, Qin Wu, Jorge Anibal Boscoboinik, Judith C. Yang,Guangwen Zhou, Atomic dynamics of gas-dependent oxide reducibility. Nature 644, 927–932 (2025). https://doi.org/10.1038/s41586-025-09394-0
Funding
This work was supported by the US National Science Foundation (Grant No. DMR 2303712). The computational modelling work was supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering (Award No. DE-SC0001135). This research used the Electron Microscopy, Proximal Probes, and Theory and Computation resources of the Center for Functional Nanomaterials and beamline 7-BM (QAS) at the National Synchrotron Light Source II, which are US DOE Office of Science User Facilities, at Brookhaven National Laboratory under Contract No. DE-SC0012704. Work at the beamline was supported in part by the Synchrotron Catalysis Consortium, US Department of Energy (Grant No. DE-SC0012335).
2025-22675 | INT/EXT | Newsroom









