Learning from Nature to Make Stronger Synthetic Polypeptides
June 18, 2025
 enlarge
enlarge
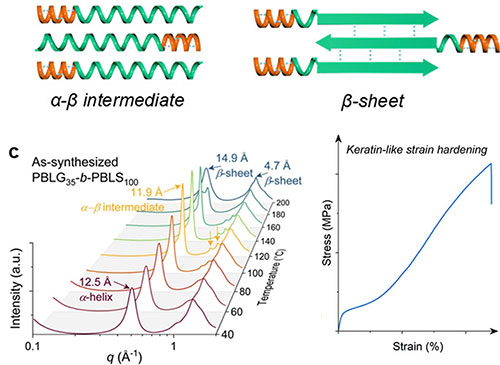
(Top) Stretched synthetic copolypeptides with Transformable PBLS or PBLC Segments. (Bottom) In situ WAXD of PBLG35-b-PBLS100upon heating shows the α-to-β structural transition with temperature. The transition results in dramatic strain hardening.
The Science
Scientists have developed synthetic polypeptides that mimic the α-to-β structural transitions of fibrous proteins from nature, achieving strain-induced mechanical reinforcement at high temperatures.
The Impact
This approach offers a new strategy for creating thermally robust, responsive, bioinspired materials.
Summary
Fibrous proteins in nature, like α-keratin, are known for their strength and flexibility, which come from their ability to shift from one molecular shape (α-helix) to another (β-sheet) under mechanical stress. Inspired by this, researchers have developed synthetic polypeptides that can undergo similar conformational changes—but at much higher temperatures than keratin can withstand.
Using a controlled polymerization process, poly(O-benzyl-l-serine) (PBLS) chains were synthesized that start in a α-helical form and can transition to β-sheet structures when heated. By compression molding at specific temperatures, they created an intermediate structural state that remains stable until mechanical strain triggers a shift to the stronger β-sheet form, leading to significant reinforcement. This strategy also works with other polypeptides bearing different side chains, such as poly(S-benzyl-l-cysteine), demonstrating robust mechanical reinforcement across a wide temperature window up to ∼ 200 °C.
In situ synchrotron X-ray scattering at the LiX and CMS beamlines at the National Synchrotron Light Source II (NSLS-II) – a U.S. Department of Energy (DOE) Office of Science user facility located at DOE’s Brookhaven National Laboratory – was used to simultaneously measure stress–strain curves and time-resolved Wide-Angle X-Ray Diffraction (WAXD) at controlled temperatures. This work confirmed the step-by-step structural changes during polypeptide deformation.
This method offers a flexible platform for developing next-generation polypeptide materials with tunable mechanical resilience and responsiveness – surpassing the temperature limitations of natural fibrous proteins and enabling potential applications demanding broad-temperature mechanical adaptability.
Download the research summary slide (PDF)
Related Links
Contact
Jianjun Cheng
University of Illinois at Urbana-Champaign
jianjunc@illinois.edu
Yao Lin
University of Connecticut
yao.lin@uconn.edu
Publications
T. Yang, J. Mao, T. Xue, S. Wu, R. Li, Y. Chen, A.A. Sitab, J. Cheng, Y. Lin. “Intermediate States Enable Keratin-like α-to-β Transformations in Strain-Responsive Synthetic Polypeptides.” J. Am. Chem. Soc. 2025, 147, 18, 15534–15544. https://doi.org/10.1021/jacs.5c02148
Funding
This work was supported by the NSF grant DMR 2210590 to Y.L. This research used resources of the CMS beamline (11-BM) and the LIX beamline (16-ID) of the National Synchrotron Light Source II through beamtime proposals (RA-316927 and BAG-310949). Both the CMS and LIX beamlines are supported by the U.S. DOE Office of Science Facilities at Brookhaven National Laboratory under Contract no. DE-SC0012704.
2025-22528 | INT/EXT | Newsroom









