Research Highlight: Watching Oxidation of Bimetallic Catalysts in 3D
innovation at CFN
April 3, 2017
What is the scientific achievement?
Bimetallic alloy nanoparticles are important catalysts because of the synergistic interaction between the metals that leads to higher catalytic efficiencies as compared to their single-metal counterparts. Understanding the oxidation mechanisms of these nanoparticles is key to optimizing their catalytic performance for fuel-cell-powered vehicles and other applications. However, it has been experimentally difficult to observe the local structural and chemical changes that occur during bimetallic oxidation because of the complexity of the 3D mosaic patterns in the nanoparticles. This work combines multiple advanced electron microscopy techniques to track the real-time oxidation of bimetallic nickel-cobalt (Ni2Co) nanoparticles on both exterior and interior surfaces.
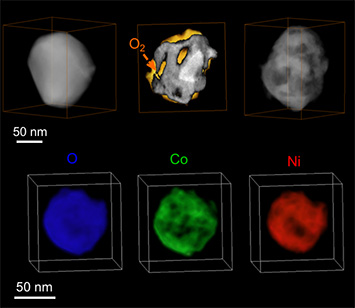
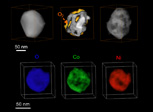
Top row: 3D evolution of a Ni2Co particle before (left), during (middle), and after (right) oxidation. Bottom row: 3D mapping of oxygen (blue), cobalt (green), and nickel (red) in a fully oxidized nanoparticle.
Why does this achievement matter?
3D reconstruction of the structure and elemental composition of the particles and real-time imaging information reveals the mechanism of bimetallic nanoparticle oxidation, which results in a Swiss-cheese-like spherical structure, in contrast to the hollow spherical structure that the monometallic parents form. The particles in the porous structure have a much high packing density than those in the hollow spheres, increasing the accessible surface area per unit packing volume—an important metric for catalyst activity. The porous particles may also make stronger structures, which would be particularly useful in applications in which mechanical specifications exclude weaker hollow structures. The ability to monitor the nanoscale structural and chemical detail of the particles undergoing oxidation on external and internal surfaces could lead to a more rational approach to designing catalysts.
This movie shows the differential distribution of cobalt, then nickel, and then the two elements combined within a single particle. Cobalt is preferentially located on the exterior surface and along the internal surfaces of pores, where it can react with oxygen (more easily than nickel does).
What are the details?
CFN Capabilities: The new FEI operando Talos transmission electron microscope/scanning transmission electron microscope in CFN’s Electron Microscopy Facility was used to collect 3D reconstruction data and real-time in situ and ex situ images of the nanoparticles as they were oxidizing. A sample holder was custom-designed to withstand the change in temperature as the nanoparticles were heated to 500 degrees Celsius and to enable tilting of the sample so that it could be scanned from every angle.
By combining real-time imaging and chemical-sensitive electron tomography, we were able to track where metal ions were reacting with oxygen to become metal oxides and where the individual elements were located within the bimetallic nanoparticles as the oxidation reaction progressed. We found that oxidation begins on the particles’ exterior surface, where pinholes are created as the metal ions (preferentially cobalt ions) move out of the particles to react with oxygen and form an oxide shell. These openings allow oxygen to access the interior surface of the particles, where oxidation continues.
Publication Reference
“Interrogation of bimetallic particle oxidation in three dimensions at the nanoscale”
L. Han1,2, Q. Meng3, D. Wang4, Y. Zhu3, J. Wang4, X. Du2, E.A. Stach1, and H.L. Xin1
1 Center for Functional Nanomaterials, Brookhaven National Laboratory, Upton, New York, 11973, United States
2 Key Laboratory of Advanced Ceramics and Machining Technology, Ministry of Education (Tianjin University), Institute of New-Energy Materials, School of Materials Science and Engineering, Tianjin University, Tianjin 300072, China
3 Condensed Matter Physics and Materials Science Department, Brookhaven National Laboratory, Upton, New York, 11973, United States
4 Key Laboratory of Material Chemistry for Energy Conversion and Storage (Huazhong University of Science and Technology), Ministry of Education, Hubei Key Laboratory of Material Chemistry and Service Failure, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan 430074, China
Nature Communications 7, 13335 (2016)
Acknowledgement of Support
This research is supported by the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. L.H. was supported by a scholarship from the China Scholarship Council (CSC) (201406250041). Q.M. and Y.Z. were supported by DOE-BES, Materials Sciences and Engineering Division under Contract DESC0012704.
2017-12170 | INT/EXT | Newsroom