Trapping Elusive Radicals in a Metal-Organic Framework
June 30, 2021
 enlarge
enlarge
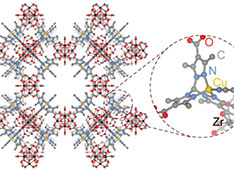
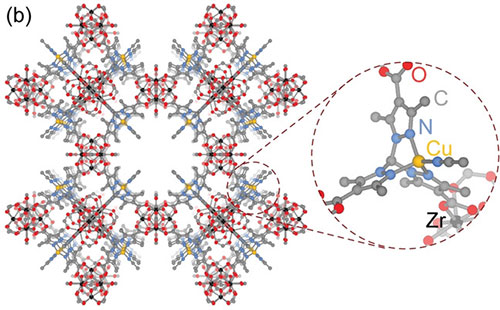
Schematic of the porous metal-organic framework showing the copper binding site (inset) used to isolate the reaction intermediate.
What is the scientific achievement?
CFN users from MIT led a collaborative team who made the first experimental observations of copper-bound anionic hyponitrite (N2O2.− ), an unusual and highly reactive radical. The team trapped the radical using a copper binding site in a metal-organic framework and observed it using several in situ spectroscopy techniques, including x-ray photoelectron spectroscopy at the CFN. They substantiated experimental observations through density functional theory simulations.
Why does this achievement matter?
Anionic hyponitrite is often proposed as a key reaction intermediate in the reductive coupling of nitric oxide to nitrous oxide, but it is extremely challenging to observe. This work demonstrates that metal-organic frameworks can isolate highly reactive species to make them observable. The strategy employed in this work can be used to isolate intermediates in enzymatic catalysis and provide key mechanistic insights.
What are the details?
Dianionic hyponitrite (N2O22−) is often proposed, based on model complexes, as the key intermediate in the reductive coupling of nitric oxide to nitrous oxide at the bimetallic active sites of heme-copper oxidases and nitric oxide reductases. In this work, the team examined the gas-solid reaction of nitric oxide with the metal-organic framework (MOF) CuI-ZrTpmC* using a suite of in situ spectroscopy techniques (density diffuse reflectance infrared Fourier transform, Raman, electron paramagnetic resonance, and ambient-pressure x-ray photoelectron spectroscopy) and density functional theory simulations. They identified an unusual chelating N2O2.− intermediate. These results highlight how site isolation in MOFs could enable the study of important reaction intermediates and provide a mechanistic scenario compatible with the proposed one-electron couple in these enzymes.
CFN Capabilities
The CFN Proximal Probes Facility was used for x-ray photoelectron spectroscopy characterization.
Publication Reference
C. Sun, L. Yang, M.A. Ortuño, A.M. Wright, T. Chen, A.R. Head, N. López, M. Dinca, “Spectroscopic Evidence of Hyponitrite Radical Intermediate in NO Disproportionation at a MOF-Supported Mononuclear Copper Site,” Angew. Chem. Inter. Ed 60, 7845 (2021).
DOI: https://doi.org/10.1002/anie.202015359
OSTI: https://www.osti.gov/biblio/1786102-spectroscopic-evidence-hyponitrite-radical-intermediate-disproportionation-mofsupported-mononuclear-copper-site
Acknowledgement of Support
This research was funded by the National Science Foundation through the Alan T. Waterman Award to M.D. (DMR-1645232). This research used resources of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. M.A.O. acknowledges the support of the Beatriu de Pinós postdoctoral program of the Government of Catalonia’s Secretariat for Universities and Research of the Ministry of Economy and Knowledge (2017-BP-00039).
2021-19085 | INT/EXT | Newsroom