- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Catalysis: Reactivity and Structure
Bridging the material gap in theoretical modeling
 The
materials gap refers to the gap between the simplified model
systems and the complex real catalysts. In general, theoretical studies
limit to deal with model systems, such as single crystal surfaces and
well-defined cluster supported on single crystal surfaces. In contrast, the
real catalysts are normally irregularly shaped particles, which are randomly
distributed on high surface materials. A significant change in fundamental
research is driven by the need to move from consideration of idealized,
model catalyst structures on single-crystal supports to more realistic
catalyst structures.
The
materials gap refers to the gap between the simplified model
systems and the complex real catalysts. In general, theoretical studies
limit to deal with model systems, such as single crystal surfaces and
well-defined cluster supported on single crystal surfaces. In contrast, the
real catalysts are normally irregularly shaped particles, which are randomly
distributed on high surface materials. A significant change in fundamental
research is driven by the need to move from consideration of idealized,
model catalyst structures on single-crystal supports to more realistic
catalyst structures.
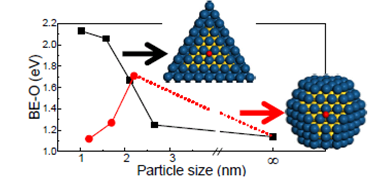
Recently, we performed DFT calculations to study the effect of size and shape on the stability and activity of Pd@Pt core-shell nanocatalysts, which has been found as active catalysts for fuel cells. According to our study, at the nanoscale (< 3nm in our case), adopting a particle model that allows us to model particles in more realistic size and shape precisely can be essential for theoretical studies to make valid connection with experiments, which has been ignored in previous studies. The stability of the nanoparticles is strongly associated with the surface contraction. For the same size, the contraction and therefore the stability depends on the particle shape. The situation is more complicated in activity that that in stability. The variation in activity with the particle size may follow different trends depending on the shape. It is attributed to the interplay between surface contraction and local structural flexibility of a particle in particular at nanoscale. The flexibility of such core-shell nanoparticles can lead to local structural changes during the reaction and therefore the in-situ study of nanoparticles under reaction conditions is essential both experimentally and theoretically. Such fundamental understanding of nanocatalysts can only be achieved by the present calculations using a fully relaxed nanoparticle model in more realistic size and shape. Our study demonstrates the importance of modeling more realistic catalysts to draw valid conclusion at nanoscale.
Ref: W. An and P. Liu, Journal of Physical Chemistry C 117 (2013) 16144-16149.




