- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Catalysis: Reactivity and Structure
Bridging the pressure gap in theoretical modeling
Pressure gap refers to the 13 orders of magnitude difference in pressure between UHV based experiments and typical operating pressures inside a catalytic reactor. A key issue in the modeling of a catalyst under operating conditions is the high coverage structures formed at high gas pressures. Although many studies have shown a correspondence between adsorption structures formed at high pressure and under UHV conditions, this is not always the case.
In collaboration with experiments, we have performed DFT calculations to determine the structures and activities of RuOx nanostructures formed on TiO2(110) under different oxygen chemical potentials. We used statistical thermodynamics to take into account the effect of temperature, oxygen pressure, and ruthenium concentration on the stability of the ruthenium oxides on the TiO2(110) surface. Our calculations showed that temperature and oxygen pressure had a dramatic effect on the elemental composition and morphology of the RuOx/TiO2(110) systems, and therefore the catalytic activities. The DFT-optimized structures agree well with the STM measurements. By adopting the unique nanostrucure, RuOx/TiO2(110) is able to display a higher activity than each individual toward CO oxidation and ethanol decomposition. Given that, to model a catalytic reaction under relatively high pressure, the effects of pressure on the catalyst phase, catalyst structure, binding energy of an intermediate and the kinetics of each elementary step have to be addressed, which may cause significant differences from the case under UHV conditions.
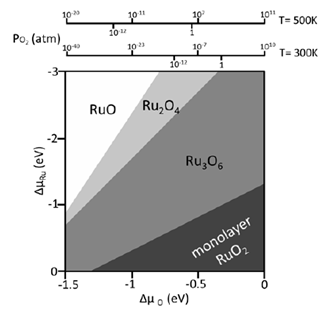
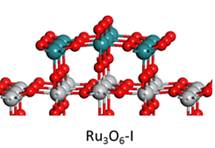
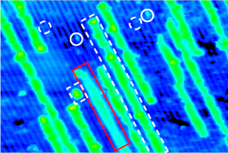
Ref: S. Kundu, A. Vidal, F. Yang, P. Ramirez, S. Senanayake, D. Stacchiola, J. Evans, P. Liu, J. A. Rodriguez, Journal of Physical Chemistry C 116 (2012) 4767-4773; F. Yang, S. Kundu, A. B. Vidal, P. J. Ramírez, S. D. Senanayake, D. Stacchiola, J. Evans, P. Liu, J. A. Rodriguez, Angewandte Chemie International Edition 50 (2011) 10198-10202; S. Kundu, A. B. Vidal, A. Nadeem, S. Senanayake, H. Idriss, P. Liu, J. A. Rodriguez, D. Stacchiola, Journal of Physical Chemistry C 117 (2013) 11149-11158.




