- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Catalysis: Reactivity and Structure
The Catalysis Reactivity and Structure group is part of the BNL catalysis science program, which advances fundamental understanding of sustainable fuel and chemical synthesis pathways with a current emphasis on catalysis for C1 chemistry such as conversion of CO2. This program pursues an improved understanding of chemical catalysis for advanced fuel synthesis and energy conversion processes by elucidating catalytically important properties over well-defined surfaces, powders and nanostructures. We study and manipulate in a systematic way a series of multifunctional catalysts which contain metal-oxide, metal-carbide or metal-sulfide interfaces. Our comprehensive research program examines the effects of size, morphology and chemical environment, providing a truly atomistic view for explaining/predicting catalytic activity.

In-situ AP-STM / AP-XPS / AP-IRRAS (model catalysts)
We prepare nanostructured materials for catalysis, focusing on the characterization and reactivity of metal-oxide nanostructures. The goal is to identify and characterize the catalytically active sites of supported nanocatalysts and to investigate the influence of particle size, morphology and support on nanostructure reactivity.
Complexities stemming from the inherent multi-component aspects of heterogeneous catalysis are explored using both ultra-high-vacuum surface science investigations of well-defined model systems, and powder diffraction and x-ray absorption studies of "real-world" systems. We bring distinct methods to common reaction themes in fuel synthesis such as hydrogen production and oxygenate synthesis.
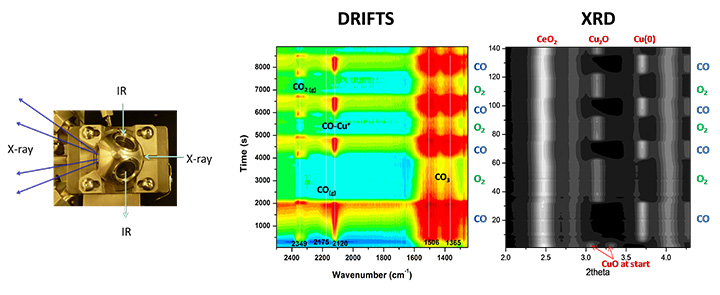
Combined In-situ XRD and Vibrational Spectroscopy DRIFTS (Powder catalyst).
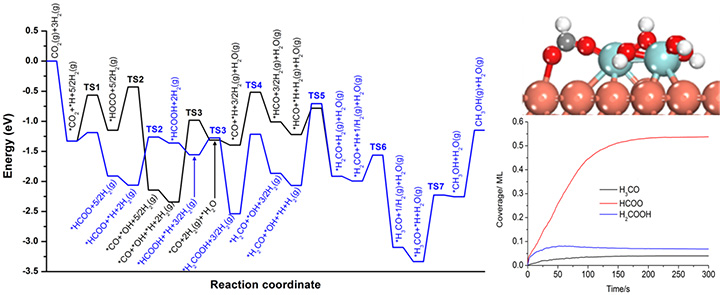
Combined DFT and Kinetic Monte Carlo (simulation).
We develop and apply new approaches for the in-situ characterization of heterogeneous catalysts using facilities available at the National Synchrotron Light Source (NSLS-II) for X-ray diffraction, X-ray absorption and ambient pressure photoemission. Quantum-chemical computation plays an essential role for basic understanding of catalytic reactions and interpretation of experimental results.
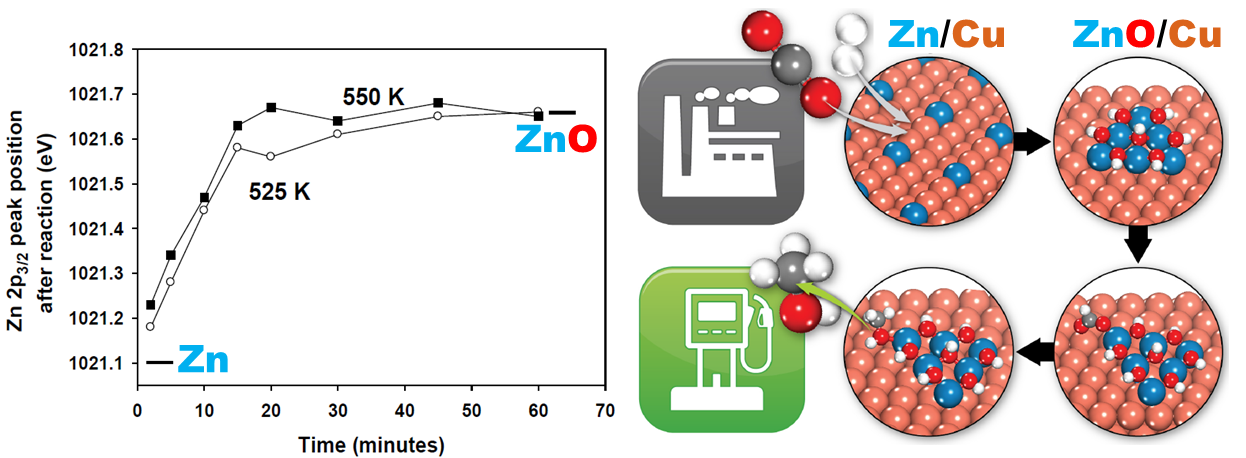
Catalysis: Active Sites for CO2 Hydrogenation to Methanol on Cu/ZnO Catalysts
We advanced fundamental understanding on a long-standing debate, the active sites of commonly used Copper/ Zinc oxide (Cu/ZnO) catalysts for carbon dioxide (CO2) hydrogenation to methanol. The transformation of surface Zn of ZnCu alloy into ZnO under the reaction condition was captured, resulting in the catalytically active ZnO-Cu interfacial sites for methanol production. Optimizing the ZnO-Cu interface should be the driving principle for designing better catalysts.
Kattel, S.; Ramírez, P. J.; Chen, J. G.; Rodriguez, J. A.; Liu, P., Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355 (6331), 1296-1299. (http://science.sciencemag.org/cgi/content/full/355/6331/1296?ijkey=e.EnqESKxFRv2&keytype=ref&siteid=sci)