- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Catalysis: Reactivity and Structure
Steam Reforming of Ethanol on Ni/CeO2: Reaction Pathway and Interaction between Ni and the CeO2 Support
The steam reforming of ethanol on a Ni-based CeO2-supported catalyst was studied using in situ X-ray diffraction (XRD), operando diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), and mass spectroscopy (MS) with a focus on the structural characterization of the catalysts, chemical identification of the reaction pathway, and understanding of the interaction between Ni and the CeO2 support. Ethoxy, acetate, carbonate, and hydroxyl species are identified by DRIFTS as surface intermediates that appear during the reaction process. The oxidation of ethoxy to acetate and the decomposition of acetate are two key steps in the steam reforming process. The CeO2 support facilitates the oxidation of ethoxy to acetate below 350 °C. Above 350 °C, the Ni metal catalyzes dissociation of the C–C bond in acetate to form carbonate and methyl, something that the CeO2 support is not able to do. The Ce(III) sites produced by the reduction of ceria in ethanol help to dissociate water forming the surface hydroxyl groups, which react with the methyl groups to produce CO2 and inhibited the methyl groups’ progress to CH4. Post-reaction transmission electron microscopy (TEM) images of the Ni/CeO2 catalyst reveal two types of carbon configurations: encapsulating carbon and filamentous carbon. A water-rich atmosphere favors formation of carbon filaments, which do not deactivate the catalyst.
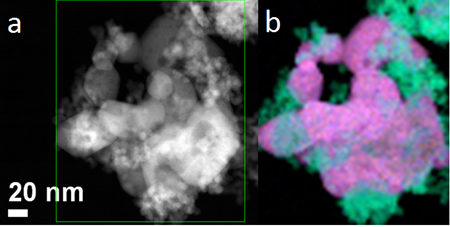
TEM image of a NiO-CeO2 catalyst used for ethanol reforming. Pink=Ni, Blue=Ce and Green=O
Ref: Xu, W.Q., Liu, Z.Y., Johnston-Peck, A.C., Senanayake, S.D., Zhou, G., Stacchiola, D., Stach, E.A., and Rodriguez, J.A. ACS Catalysis, 2013. 3(5): p. 975-984. DOI: 10.1021/cs4000969




