- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Artificial Photosynthesis
Calculation of Thermodynamic Hydricities and the Design of Hydride Donors for CO2 Reduction
We have investigated through DFT calculations the hydride donating power, or “hydricity” of various ruthenium- and rhenium-based complexes that incorporate the pbnHH (pbnHH = 1,5-dihydro-2-(2-pyridyl)-benzo[b]-1,5-naphthyridine) ligand to model the function of NADPH. These visible-light-generated, photocatalytic complexes produced by the disproportionation reaction of a protonated-photoreduced dimer of a metal-pbn complex may be valuable for use in reducing CO2 to fuels such as methanol. The further one-electron reduction of the previously characterized isomeric [Ru(bpy)+(pbnHH)]2+ to form the [Ru(bpy)2·–(pbnHH)]+ species, where (bpy)2·– denotes one electron distributed over both bpy ligands, shows promise for reduction of metal-bound carbonyl ligands to produce the bound formyl anion and beyond. Those species donate hydrides to produce the pbnH· intermediate, which has been shown to form the active species pbnHH through a disproportionation reaction. Experimental evidence is presented that the excited state lifetime of photoexcited [Ru(bpy)2(pbnHH)]2+ is ca. 70 ns, and that this excited state can be reductively quenched by triethylamine or 1,4-diazabicyclo[2.2.2]octane (DABCO) to produce the one-electron-reduced [Ru(bpy)2(pbnHH)]+ species with lifetime exceeding 50 μs, thus opening the door to new opportunities for hydride-transfer reactions leading to CO2 reduction by producing a species with much increased hydricity. Calculations indicate that the triply-reduced, doubly-protonated ruthenium complex is sufficiently “hydridic” to reduce a metal-bound carbonyl on CpRe(NO)(CO)2.
[Ru(bpy)2·–(pbnHH)]+ + CpRe(NO)(CO)2 → [Ru(bpy)2(pbnH·)]2+ + CpRe(NO)(CO)(CHO)
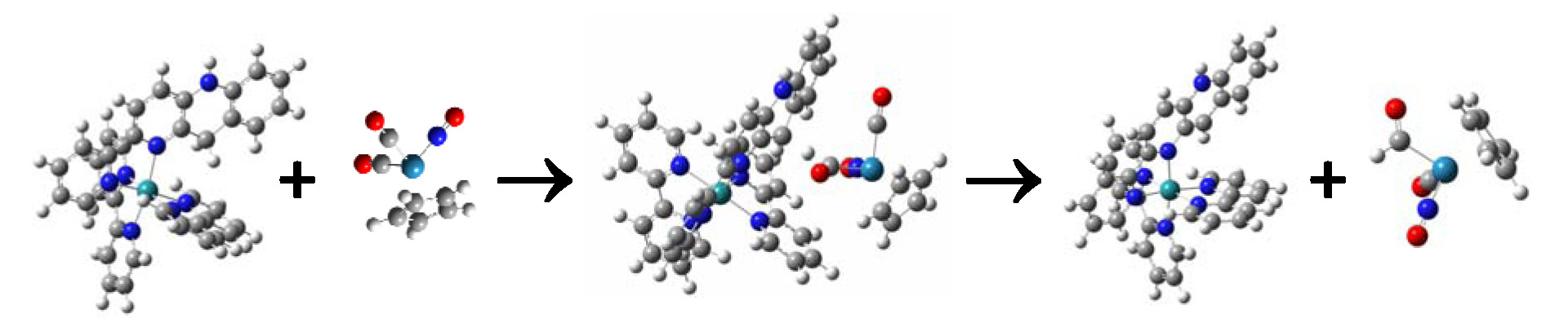
The calculated transition state (TS, middle of figure) for hydride transfer from the [Ru(bpy)2·–(pbnHH)]+ complex to CpRe(NO)(CO)2 to form CpRe(NO)(CO)(CHO) and [Ru(bpy)2(pbnH·)]2+.
Proc. Nat. Acad. Sci. USA 2012, 109, 15657-15662, DOI: 10.1073/pnas.1201026109.




