- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Electron- and Photo-Induced Processes
The Marcus Inverted Region for Bimolecular Electron Transfer
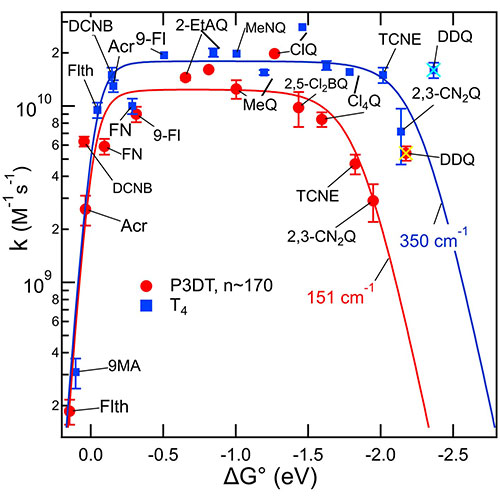
Rate constants for bimolecular electron transfer (ET) increased with driving force, −ΔG°, reached a plateau, and then decreased in an inverted region. This rate data was described well by electron transfer theory subject to a diffusion-controlled limit. These were for ET from radical anions of polydecylthiophene (P3DT) to a series of acceptors in THF solution. When the donor was the smaller anion of quaterthiophene (T4•-) the inverted region was much less prominent and still less so for when the donor was the anion of bithiophene (T2•-). Description of the data using ET theory identifies smaller electronic couplings for the highly delocalized P3DT anions as enabling the inverted behavior: The presence of a Marcus inverted region is a consequence of delocalized electronic states. The results further imply that electronic couplings smaller than usually found for molecules in contact could boost efficiency of energy storage by electron transfer and identifies size-mismatch as an important concept in control of electronic couplings.
J. Am. Chem. Soc., 2020, 142, 17997 DOI: 10.1021/jacs.0c04780




