- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events
Electron- and Photo-Induced Processes
Creating super-oxidants with pulse radiolysis
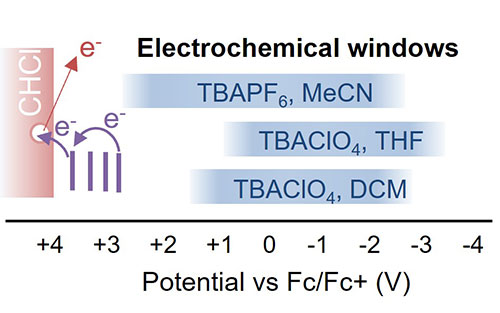
PR forms super-oxidants by ionizing the solvent, CDCl3, (scattered electron in red) leaving a solvent-formed radical cation ‘hole’ (white circle) that oxidizes solutes (violet energy levels) to form super-oxidants, beyond what is possible with electrical injection of charge in the typical electrolyte/solvent combinations required in electrochemistry (blue bands).
Pulse radiolysis (PR) enables the full redox window of a solvent to be accessed, as it does not require electrodes or electrolyte which limit the potentials accessible in voltammetry measurements. PR in chloroform has the additional possibility to enable reaching highly positive potentials because of its large ionization potential (IP). PR experiments demonstrated the formation of the (deuterated) chloroform radical cation CDCl3+˙, identifying it as the source of the broad absorption in the visible part of the spectrum. Results indicated that solutes with a redox potential up to +3.7 V vs. Fc/Fc+ can be oxidized by CDCl3+˙, which is far beyond what is possible with electrochemical techniques. Oxidation is not efficient because of rapid geminate recombination with chloride counterions, but also due to rapid decomposition of CDCl3+˙ which limits the yield of otherwise longer-lived free ions. The rapid, 6 ± 3 ns, decomposition, confirmed by two independent experiments, means that a solute must be present at a concentration >100 mM to capture >90% of the free holes formed. Addition of ethene removes the broad, overlapping absorptions from ubiquitous (chlorine atom, solute) complexes created by PR in halogenated solvents enabling clear observation of solute cations. The results also unravel the complex radiation chemistry of chloroform including the large reported value G(–CHCl3) = 12 molecules/100 eV for the decomposition of chloroform molecules.
Phys. Chem. Chem. Phys., 2020, 22, 14660 DOI: 10.1039/D0CP01948H




