- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Electron- and Photo-Induced Processes
Fundamental Chemistry of Ionization
Fast electrons produce ions and excited states in ways that a well understood in water and less well understood in organic fluids, ionic liquids and polymer films.
Step Capture of Electrons and Holes Electrons or holes produced suddenly in condensed media attach to molecules in processes characterized by rate constants, but some electrons (holes) attach much faster than the 15 ps time resolution of the OFSS detection system. That this can occur for holes as well as electrons is especially surprising; it’s nature and extent is under investigation. Step capture occurs in ionic liquids.
Production of Excited States Excited states can be produced by direct excitation and production by recombination of electrons and holes is well known in nonpolar liquids. In moderately polar liquids the recombination can limit formation of singlet states but the energetics are not well understood.
Fast Formation of Triplet Excited States Pulse radiolysis is one of the best methods to create triplet excited states but the process is normally slow. Geminate recombination of electrons and holes formed together is expected to produce singlet excited states, but there is some fast formation of triplets.
Highly Mobile Charges in Liquids and Supercritical Fluids Electrons display mobilities as large as 100 cm2/Vs in some liquids as determined by pulse radiolysis with dc conductivity. Mobilities vary dramatically near the critical point of supercritical fluids.
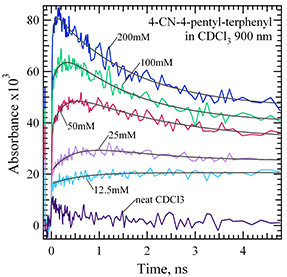
Transient absorption using LEAF’s OFSS showing “step” capture of holes
by a terphenyl derivative in CDCl3 ionized by electron pulses.




