Leveraging DNA to Create Advanced, Usable Materials with Jason Kahn
interview with a CFN staff member
May 21, 2021
 enlarge
enlarge
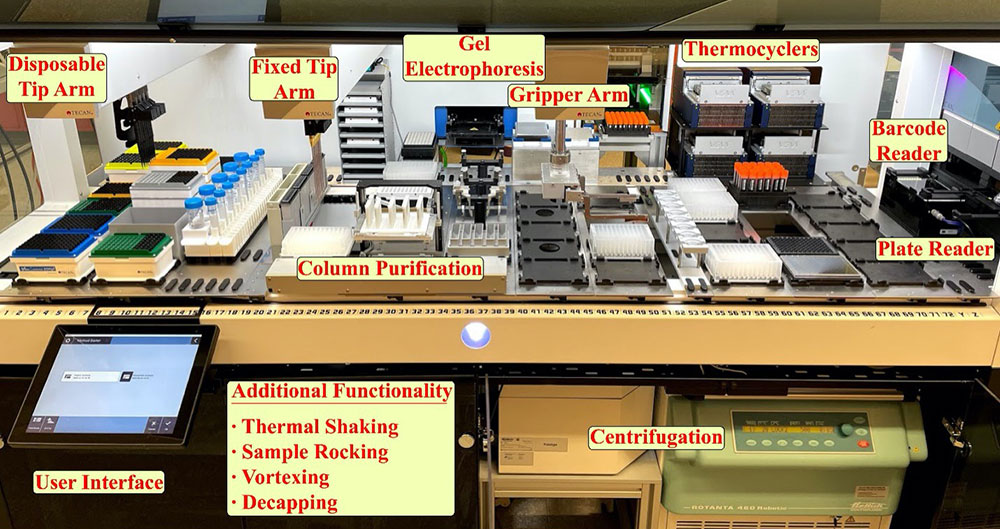
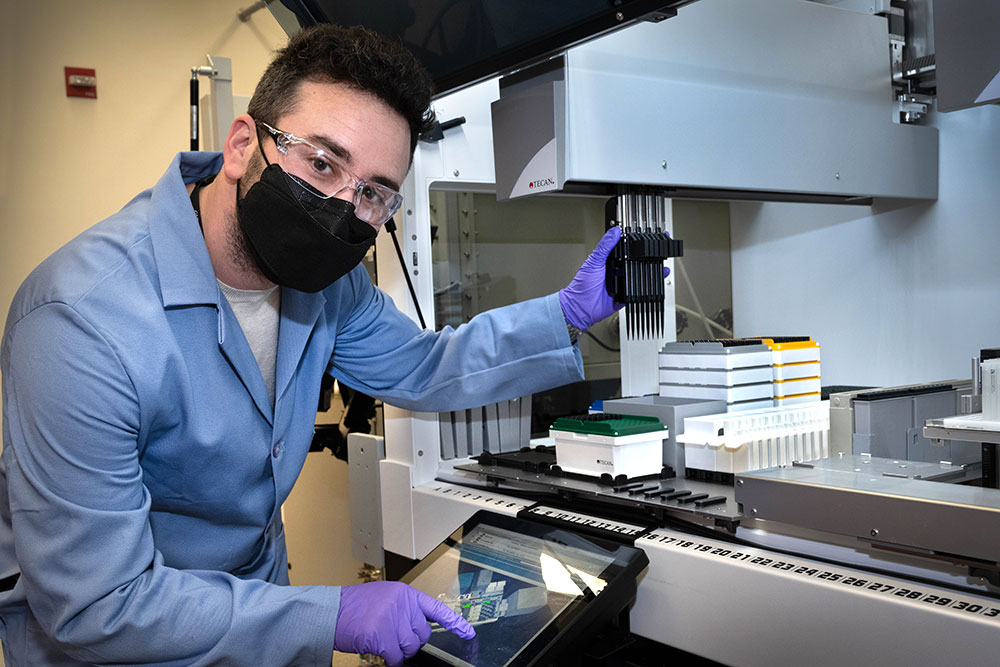
Jason Kahn inside a lab at the Center for Functional Nanomaterials, where he's building a one-of-a-kind liquid-handling robot. Users of this robot will be able to synthesize, purify, and characterize materials in a consistent manner to explore self-assembly processes. Machine learning will feed back information into these processes, enabling users to assemble desired organizations.
DNA is a biomolecule containing the genetic instructions for living things to develop and function. It’s also a versatile building block for constructing precisely ordered structures of useful materials. That’s because DNA can be programmed to self-assemble in desired ways based on the specific rules of complementary base pairing. The Soft and Bio Nanomaterials Group at the Center for Functional Nanomaterials (CFN)—a U.S. Department of Energy (DOE) Office of Science User Facility at Brookhaven National Laboratory—is a leader in the bottom-up assembly of functional materials using DNA. Since joining the group in December 2019, staff scientist Jason Kahn has not only been conducting research in DNA-based assembly but also building a one-of-a-kind automated platform to explore self-assembly processes. He brings expertise in integrating DNA with other classes of materials from postdocs at Columbia University and The Hebrew University of Jerusalem in Israel. He received a PhD in biological engineering with a concentration in materials science and a bachelor’s in biological engineering from Cornell University.
How did you come to join the CFN?
I’m from the Long Island area, so I grew up knowing about Brookhaven Lab. When I was a kid, my parents took me to see Brookhaven’s weather station. I also visited Brookhaven through school programs.
 enlarge
enlarge
Jason Kahn (center) in a lab at Columbia University during his postdoc, with PhD students Yan Xiong (left) and Brian Minevich (right) and Columbia Engineering professors Sanat Kumar (second from left) and Oleg Gang (fourth from left). Note: This photo was taken prior to current COVID-19 social distancing guidelines.
In graduate school, I was accepted to the DOE Office of Science Graduate Fellowship Program. In addition to funding my research and providing a monthly stipend, the program brought me and a cohort of other graduate students to different national labs around the country. These tours gave me a greater introduction to Brookhaven and the national labs at large.
After receiving my graduate degree, I did a postdoc in Israel. Coming back to the United States to continue my research, I found Oleg Gang at Columbia to be an ideal match, both because of his lab’s research and the opportunity for me to get introduced to the national lab setting once again. In addition to being a professor at Columbia, Gang is the leader of the CFN Soft and Bio Nanomaterials Group. Over the years of my postdoc at Columbia, I developed closer collaborations with Brookhaven. When a scientist position opened up at the CFN, I saw the opportunity to develop new directions and concepts in materials assembly and applied.
What did your postdoc work focus on, and how does it relate to the research you’re now doing at the CFN?
To broaden the scope of my research beyond my PhD work, which was generally focused on DNA nanotechnology, I was looking to gain further background and experience in chemical methods. During my postdoc in Israel, I continued working in the area of DNA nanotechnology but began incorporating different chemistries to associate DNA-based materials with other classes of materials such as polymers, metal nanoparticles, and enzymes. In Oleg’s lab, I took this concept further by prescribing three-dimensional order into the DNA self-assembly process using specific nanoscale structure. These two postdocs helped define my current research, where I’m combining DNA and other materials to assemble them into functional organizations for real-world applications.
DNA is often called the “molecule of life.” How can it be used to assemble functional materials?
 enlarge
enlarge
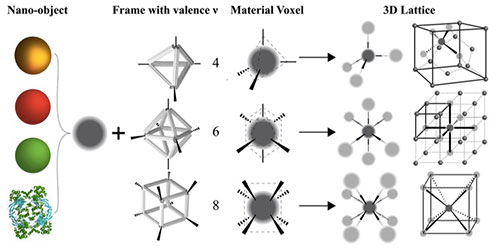
A schematic of the platform for synthesizing material voxels by incorporating various classes of nano-objects into DNA origami frames that define binding characteristics, enabling control of material self-assembly. For example, tetrahedral DNA frames assemble into diamond structures, octahedral into simple cubic, and cubic into body-centered cubic. Research published in Nature Materials (2020).
Though most of us think of DNA as purely a biological material, it’s really just a chemical polymer, or a large molecule made of many repeating smaller units. In our group, we use synthetic DNA to synthesize very specific nanostructures with different shapes and binding topologies—“DNA origami”—and chemically associate them with a particular material. We use the specificity of DNA interactions to then bind those structures, which are coupled between the DNA and material, together in a controlled manner. We call the combination of a material with this DNA frame a “material voxel,” where we have control over a nano-object’s binding valence, coordination, and specificity. Through structure designability and interaction specificity, we try to introduce organizational complexity into the self-assembly process. Our research questions revolve around how to do this, as there’s always a balance between the amount of specificity needed to generate the desired organization and the amount of specificity that interferes with assembly kinetics.
In the past, DNA materials have been viewed as just a way to study these kinds of systems. But what we’re seeing through recent publications and notably our work is the ability to use these DNA materials in functional systems. We can stabilize these constructs or convert a full biomaterial-based system into to an inorganic system with useful organizations for biomedicine, industrially relevant catalysis, energy storage, optics, and many other applications. The idea that because we originally built something with DNA it’s always stuck in this biomaterial realm is no longer a limitation. We not only can transform DNA into another stabilized material but also make the stabilizing material functional. For example, our group recently showed how 3-D DNA lattices could be coated with silicon dioxide and converted into superconducting lattices, which conduct electricity without resistance. In another project, our group demonstrated how a peptide-like molecular coating could stabilize 3-D DNA nanostructures in biomedically relevant environments, where they would otherwise be destroyed.
Compared to other synthesis methods, what makes the DNA origami approach unique?
Our DNA-based platform offers a way to achieve organizations not achievable through other means. Top-down assembly has several limitations in building out 3-D organizations in terms of scale, diversity of materials, and throughput. For example, you can do a top-down lasering of a desired structure, but then you have one specific structure made from one material, as opposed to 10,000 in a single-tube reaction with the ability to incorporate multiple material classes. So, top-down fabrication is not really suited for bulk output of highly ordered 3-D assemblies. Other bottom-up methods of creating 3-D order don’t have the specificity to introduce different classes of materials in desired spaces or orientations.
How have you been applying the DNA-based platform to your research at the CFN?
My research encompasses both the use of this DNA-based platform to construct functional materials and further development of the platform itself. For example, I’m looking at how the platform can be used to organize enzymes in meaningful ways to control chemical outputs. In this way, we can gain control over biological catalysis (biocatalysis). We’re increasingly seeing that many chemical methods can be supplanted by more biologically oriented mechanisms. Given larger societal concerns over sustainability, it is very worthwhile to consider how we can organize biological components to provide alternate methods of producing chemicals and energy.
Enzymes are the workhorses of the biological world, the functional components of biological systems. The idea here is to create a DNA origami–based enzyme “nanofactory” capable of churning out high-value products from low-cost substrates—for example, turning monomers of sugars into complex sugars, and perhaps later biofuels. Such a nanofactory could also be useful for synthesizing certain classes of molecules, particularly those with a chirality (twist or handiness) to them. Chiral molecules are very difficult to synthesize chemically, but biology handles such complexity very well. The specificity of biological reactions and interactions allow for specificity in product synthesis, which is particularly important in manufacturing pharmaceuticals.
I’ve also been looking at how to explore organizational complexity using binding specificity and structure. Suppose you have a cube with 10,000 components, and you want a specific organization of materials in this cube. The best way to design the cube isn’t to specifically encode all 10,000 points; it’s to find some minimal way of adding assembly instructions to the self-assembly process so that you get the assembly you desire without needing to fully define the system. As I mentioned before, there’s always a tradeoff between how specific you want an object and where that level of specificity interferes with and destroys the ability to assemble it in the first place. What I’m trying to do is find a minimal set of points in 3-D space that can be repeated to create an overall structure. What I determine theoretically may not end up being an optimal assembly pathway in practice, and thus a system needs to be developed to modify assembly pathways by actively combining theory and experiment.
In addition to your hands-on research, you’re also building an automated platform to explore self-assembly processes. What will this platform do?
The liquid-handling robot will combine synthesis, purification, and characterization of materials and use machine learning to feed back information into the assembly process. The idea is that somebody can have a specific design in mind and come to use our various classes of nanomaterials—such as DNA, nanoparticles, and enzymes—to functionally explore that assembly space and construct the desired organization.
Our platform is really one of a kind due to its focus. Other liquid-handling robots exist, but they’re not as comprehensive. For example, at other national labs, some robots have been oriented toward particle synthesis. The set of instruments associated with our robot allows for a step forward in combining synthesis and characterization in a system that’s able to then iterate on its own assembly process. Connecting all these aspects will bridge the gap between unknown material assembly processes and desired material structure. This platform will be continually evolving, incorporating new characterization tools as we move forward.
The platform will both accelerate research and allow users to produce and characterize materials in a controlled, consistent manner. As careful as someone is, variability is inevitable. If we’re exploring a parameter space, we need everything to be as consistent as possible; this consistency involves not only the volumes of materials and the way they are pipetted but also the time between steps.
With an automated liquid-handling setup, standardized protocols in place, material classes on hand, the ability to barcode and store different assemblies, and software that links experimental design to the materials and robotic instrumentation, we create the possibility of becoming a center for people to both engage with our research and combine their classes of materials with our methodology. I’m really excited to work with users and collaborators from dramatically different fields to create assemblies that have never been produced before and investigate potential breakthroughs in applications and functions that have never been tested before.
A liquid-handling robot under development at the Center for Functional Nanomaterials will combine synthesis, purification, and characterization of materials and use machine learning to feed back information into material assembly processes to construct the desired organizations.
How far along are you in constructing this platform?
It’s in the commissioning phase. Over the past year, I’ve spent a lot of time incorporating function from the various connected instruments. When you combine different components from different companies for different purposes, you create this ecosystem of interaction, and you need to gain a deep understanding to create a system that acts in sync.
Most of the system’s functionality is now up and running. I’m at the point of considering what the connections are between synthesis, purification, and characterization to conduct a set of experiments as opposed to a single experiment. There are many parameters to consider in self-assembly, so I need to connect the results of self-assembly processes to our libraries of materials, interactions, and structures. In other words, completing the loop (synthesis, purification, and characterization) and coming back to the starting materials with a sense of which assembly pathways were and weren’t efficient and which materials and interactions should be selected for the next iteration.
To handle the large parameter space, we also need to incorporate theory, modeling, and machine learning into our analysis and experimental design. To help integrate experiment with theory and modeling, we’re currently engaging with our internal CFN Theory and Computation Group and plan to collaborate with other departments at Brookhaven, especially the Computational Science Initiative, as well as university groups, in the future.
Though you’ve had interactions with national labs previously, what’s it like working at one of them and specifically at a user facility?
When I first joined the CFN, I was a bit intimidated. Everyone is really an expert not just in their fields, as you would expect, but also on their specific instruments. I feel this aspect is unique to the national lab environment and user facility setting. Often in academic environments, there’s a lot of equipment and expertise floating in and out. At the CFN, you have instruments coupled with long-lasting expertise. This coupling greatly aids user projects because users can potentially reach out to multiple CFN scientists for guidance on the types of methods to use, both to create the systems they want and to analyze them.
I now feel like a fuller member of the Brookhaven community. I see many resources and opportunities that I look forward to leveraging as I move forward in my career. While I’m focusing on my particular automated approach, there are other projects I hope to tie into. Many researchers at CFN and across other departments at Brookhaven are trying to integrate automated experimentation into their material platforms. For example, CFN group leader Kevin Yager is developing machine learning capabilities for materials characterization and experimentation using block copolymer materials, and I hope to develop powerful new methodologies centered around the unique self-assembly capabilities of DNA-based systems. The biggest hurdle is integrating everyone’s work into systems that are friendly with each other. The goal is not to make isolated instruments but to create cross-cutting platforms we can share and build upon. When people look at Brookhaven from the outside, what they should hopefully see crystallizing is a broader push to define new methods of manufacturing functional materials.
When did you become interested in science and in particular biological engineering?
My interest in science was first based in earth science. When I was very young, I also loved looking at globes and maps. As I grew older and noticed how the world around me is filled with active and complex natural processes and structure, I began to wonder: is there a way to tap into these mechanisms in nature to provide function for ourselves? As a child, I thought about tapping into the chemical energy that plants produce and converting it into something directly usable, such as electrical energy. This guided me toward biological engineering. While I don’t necessarily have this direct research focus now, it broadened to say, in the biological realm, how are systems assembling? How can we use the specificity of biological systems to inspire—and, in our case, directly use these biomaterials—to produce function?
Brookhaven National Laboratory is supported by the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https://energy.gov/science.
Follow @BrookhavenLab on Twitter or find us on Facebook.
2021-18906 | INT/EXT | Newsroom