Chemists Prove the Existence of LiH in Battery
Researchers used ultrabright x-rays to identify lithium hydride and a new form of lithium fluoride in the interphase of lithium metal anodes
March 31, 2021
 enlarge
enlarge
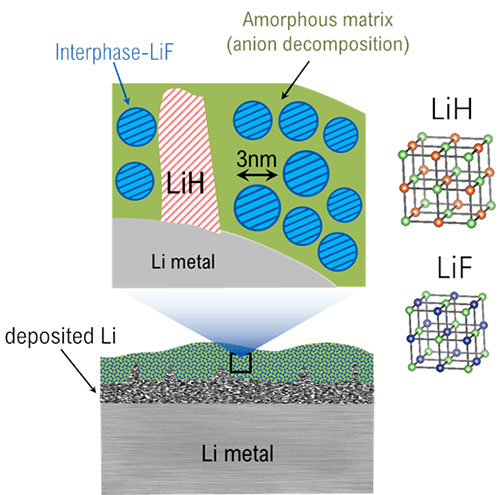
The schematic depicts the location within the solid-electrolyte interphase (SEI) where LiH and LiF can be found as well as the different crystalline structures of both. Image credit: Nature Nanotechnology 16, 549-544 (2021)
The Science
Scientists identified the existence of lithium hydride (LiH) in the solid-electrolyte interphase (SEI) on lithium metal anodes, showing ways to differentiate between LiH and lithium fluoride (LiF).
The Impact
Batteries with lithium metal anodes offer a higher energy density but they are currently not rechargeable. This study identified key components of the SEI and resolved a controversial issue regarding the existence of LiH in the interphase, which influences the reaction mechanism.
Summary
Conventional lithium-ion batteries can be found in a variety of electronics, from smartphones to electric vehicles. While lithium-ion batteries have enabled the widespread use of many technologies, they still face challenges in powering electric vehicles over long distances. To build a battery better suited for electric vehicles, researchers are focusing on batteries made with lithium metal anodes. These batteries offer more than double the energy density of regular lithium-ion batteries with traditional graphite anodes. However, batteries based on lithium metal anodes lack “reversibility,” the ability to be recharged through a reversible electrochemical reaction.
In this study, scientists presented an essential step in making these batteries reversible by revealing critical new insights into the solid-electrolyte interphase (SEI). The SEI is a very thin solid material layer that forms on the battery’s electrode during the electrochemical reaction and is the key to reversibility. To study the thin SEI layer, the team of scientists turned to the X-ray Powder Diffraction (XPD) beamline at National Synchrotron Light Source II (NSLS-II). As a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory, NSLS-II offers a wide range of x-ray scattering and spectroscopy tools, such as the XPD beamline.
Their detailed investigation of the SEI showed exciting results; LiH is identified and differentiated from LiF. The scientists used a technique called Rietveld refinement to characterize the crystalline structure, and thus could show the difference between LiH and LiF. Furthermore, they verified their findings through an air exposure experiment in which the researchers saw the expected decrease of LiH that is not stable in air compared to LiF. In addition, the team also identified the structural differences between LiF in the SEI and LiF in the bulk, with the former having unique properties that facilitate lithium-ion transport through the SEI. They also identified amorphous phases using pair distribution function analysis.
Researchers expect that the results of this study will provide much-needed practical guidance on lithium metal anodes, propelling research on this promising material forward.
Download the research summary slide
Related Links
Press Release: “Chemists Settle Battery Debate, Propel Research Forward”
Contact
Jie Xiao
Pacific Northwest National Laboratory
jie.xiao@pnnl.gov
Xiao-Qing Yang
Chemistry Division, Brookhaven National Laboratory
xyang@bnl.gov
Enyuan Hu
Stony Brook University
enhu@bnl.gov
Publications
Z. Shadike, H. Lee, O. Borodin, X. Cao, X. Fan, X. Wang, R. Lin, S.-M. Bak, S. Ghose, K. Xu, C. Wang, J. Liu, J. Xiao, X.-Q. Yang, E. Hu. Identification of LiH and nanocrystalline LiF in the solid–electrolyte interphase of lithium metal anodes. Nat. Nanotechnol. (2021). DOI: 10.1038/s41565-020-00845-5?
Funding
The work done at Brookhaven National Laboratory was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Vehicle Technology Office of the US Department of Energy (DOE) through the Advanced Battery Materials Research (BMR) Program, including Battery500 Consortium under contract no. DE-SC0012704. The work done at Pacific Northwest National Laboratory was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US DOE through the Advanced Battery Materials Research (BMR) Program (Battery500 Consortium) under contract no. DE-AC02-05CH11231. The work at the Army Research Laboratory was performed under JCESR, an Energy Research Hub funded by Basic Energy Sciences, US DOE. This research used beamline 28-ID-2 of the National Synchrotron Light Source II, a US DOE Office of Science user facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704.
2021-18941 | INT/EXT | Newsroom









