X-rays Reveal Electronic Details of Nickel-based Superconductors
Findings show similarities to and differences from cuprate superconductors, including more complex electronic structure
April 17, 2023
 enlarge
enlarge
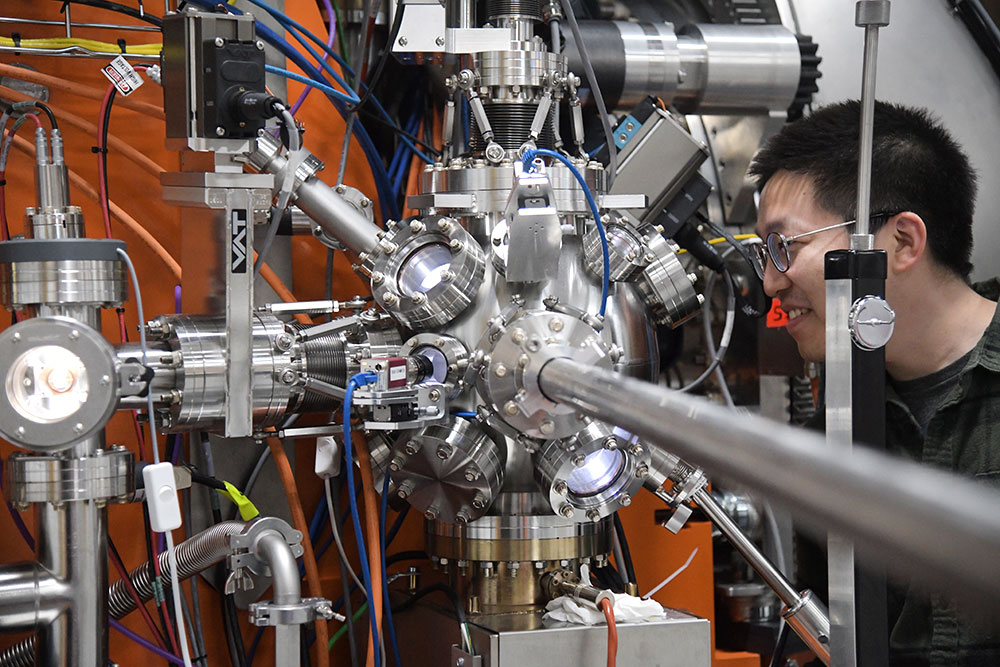
Yao Shen, a postdoctoral researcher at Brookhaven Lab and first author of two papers describing the electronic structure of a nickel-based superconductor, at the SIX beamline of the National Synchrotron Light Source II (NSLS-II) where the experiments were done.
Scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory have discovered new details about the electrons in a nickel-based family of superconducting materials. The research, described in two papers published in Physical Review X, reveals that these nickel-based materials have certain similarities with—and key differences from—copper-based superconductors. Comparing the two kinds of “high-temperature” superconductors may help scientists zero in on key features essential for these materials’ remarkable ability to carry electrical current without losing energy as heat.
“The quest to understand high-temperature superconductors is a decades-old challenge,” said Mark Dean of Brookhaven Lab’s Condensed Matter Physics & Materials Science Department, who led the research described in both papers. Ever since copper-based, or cuprate, superconductors were discovered in the 1980s, scientists have been trying to understand what makes them tick.
The interest is driven in large part by their potential for energy-saving applications. Picture power lines that deliver electricity to homes far from wind and solar farms without losing a speck of energy, and computers and other devices that function flawlessly without the need for expensive and energy-intensive cooling.
Trouble is, despite their “high-temperature” moniker, cuprate superconductors themselves must be kept extremely cold to operate—well below zero degrees Fahrenheit. Discovering what allows electrons in these materials to overcome their normal “like-charge” repulsion and flow with no resistance could perhaps point the way to superconductors that operate in closer to real-world conditions.
“These materials are also a testbed for efforts to understand other quantum materials where electrons interact very strongly,” said Steven Johnston, a theorist at the University of Tennessee and a coauthor of the paper. “You could make a reasonable argument that this is the most important open problem in the physics of materials.”
 enlarge
enlarge
Members of the research team at the NSLS-II SIX beamline, left to right: Johnny Pelliciari (SIX beamline scientist), Yao Shen, Mark Dean, and Wei He (all of the Condensed Matter Physics & Materials Science Department).
Nickel analogs
As part of the quest to crack the case on cuprates, scientists have looked for analogs—similar superconducting compounds they could study and compare to give them clues for improving properties.
“Maybe, if you just tweak something, you can make a property such as the temperature of the transition to superconductivity higher, or you can make materials with cheaper elements for applications,” said Yao Shen, a postdoctoral researcher at Brookhaven and first author of the publications.
Nickel was a logical choice. Its proximity to copper on the periodic table implies that compounds made from these next-door-neighbor transition metals might operate in similar ways but with enough differences to point out what is essential to superconductivity.
But even before scientists at Stanford University successfully created a nickel-based superconductor in 2019, others wondered whether the nickel compounds could be considered true analogs to cuprates. Once the nickelates were synthesized, the quest to find out began.
“Seeing” electronic behavior
These studies used x-rays at Brookhaven Lab’s National Synchrotron Light Source II (NSLS-II), a DOE Office of Science user facility that enables research on the microscopic structure, chemistry, and other properties of all sorts of materials. The team used the Soft Inelastic X-Ray (SIX) beamline, run by study collaborators Valentina Bisogni and Jonathan Pelliciari, to compare the electronic properties of a layered nickelate superconductor (La4Ni3O8) with those of a well-known cuprate (La2−xSrxCuO4).
 enlarge
enlarge
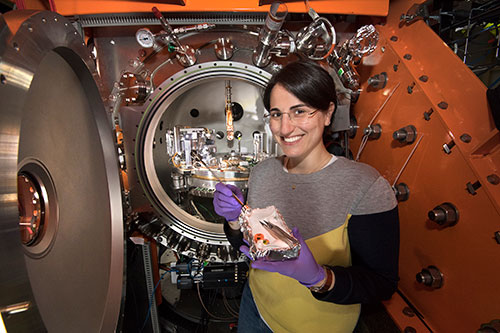
NSLS-II SIX beamline scientist Valentina Bisogni, a coauthor on this research, stands in front of the chamber where samples are placed for analysis by x-rays.
They wanted to know which electrons (from which elements) in each compound contribute to superconductivity and other electronic properties, including the presence of a “charge-density wave.” This ordered pattern of electrons might play a role in generating the material’s superconductivity.
“Scientists have evidence that superconductivity in cuprates is associated with very strong magnetic interactions between the copper ions,” said Michael Norman, a collaborating scientist from Argonne National Laboratory. “So, in addition to comparing the electrons involved in superconductivity in these two materials, we also wanted to look for evidence of magnetic interactions between the nickel ions in these nickelates and understand which elements contribute electrons that form both the charge and magnetic density waves in these materials.”
The SIX beamline, with its world-leading energy resolution, allows the scientists to “see” these subatomic scale details by tuning the x-ray energy precisely to the individual elements in the sample using a technique called resonant inelastic x-ray scattering (RIXS).
“We can tune our x-ray energy to resonate with either the oxygen or nickel or other elements and then we can see the electronic properties of those specific elements,” Dean said. “We used that alongside theory calculations to get a detailed picture of how these materials work electronically.”
Key similarities and differences
The findings indicate substantial similarities between the nickelate and cuprate superconductors—and some differences.
For example, the scientists found that in both sets of materials, the transition metal (copper or nickel) and oxygen both contribute to the materials’ electronic properties, but the magnetic interactions among nickel atoms, mediated by intervening oxygens, are slightly weaker than the oxygen-mediated magnetic interactions among copper atoms in the cuprates.
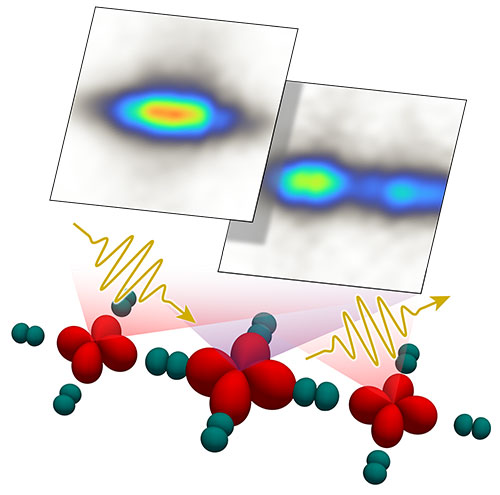 enlarge
enlarge
This cartoon shows how x-rays (yellow zigzags) reveal information about atoms' electronic structure in a nickel-oxide superconductor, where nickel atoms have red electron orbitals and oxygen-atom orbitals are teal. The larger the orbital, the more electrons in it. These studies are helping researchers understand similarities and difference between nickel- and copper-based superconductors.
“Cuprates have this very nice well-aligned energy between the copper and the oxygen, and that’s why they are very strongly magnetic,” Shen said. “A similar thing happens in the nickel compounds just to a slightly less perfect extent.”
The scientists found some key differences in the electronic properties that contribute to the generation of charge order—the charge density wave—in the two classes of superconductors. It turns out that the charge density wave in the nickelate is much more complex than that of the cuprate, coming from the combined interactions of all the different elements in the material.
“These findings indicate that the nickel compounds are promising to learn more about how the cuprates work, and they indicate the different ways you might want to change the nickel compounds to make them more like the cuprates—to have stronger magnetism or stronger superconductivity,” said Jennifer Sears, a postdoctoral researcher at Brookhaven.
“X-rays are really showing their power in probing these types of problems. The capabilities at NSLS-II have made it possible for us to work out this physics quite quickly in a way that wouldn’t have been the case without this new generation of RIXS instruments,” noted collaborator Matteo Mitrano of Harvard University.
Next steps include exploring the contributions of the rare-earth elements—lanthanum, strontium, and others—to the properties of these materials.
“The rare earth layer is not thought to be electronically active in the cuprates, but that’s an open question in the nickel-based materials,” Dean said.
The tools at NSLS-II will make it possible to explore that question, too.
Additional co-authors on this work included John Mitchell of Argonne National Laboratory, who together with Junjie Zhang provided the material samples that were prepared using unique high pressure crystal growth techniques, and x-ray spectroscopy specialist Gilberto Fabbris, who is also based at Argonne. The research and the facilities used were funded by the DOE Office of Science (BES).
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Follow @BrookhavenLab on Twitter or find us on Facebook.
2023-21189 | INT/EXT | Newsroom