Light Teaches (Co)Enzymes New Tricks
Scientists investigated the reaction of a specific class of enzymes to light
July 31, 2019
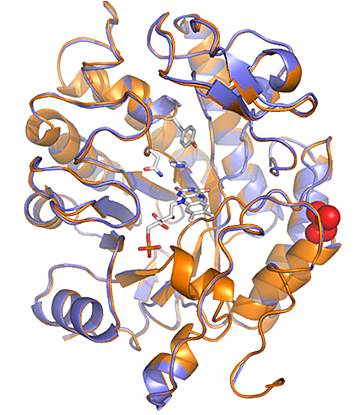
Superimposed x-ray crystal structures of GluER-T36A (blue) and GluER (orange) with the T36A mutation highlighted in red. Despite having a structure indistinguishable from wild-type GluER, GluER-T36A provides a higher product yield. Image courtesy of Science 364, 1166–1169 (2019).
The Science
A suite of flavin-dependent enzymes were shown to catalyze the radical-based production of nitrogen-containing cyclic molecules when exposed to light.
The Impact
Using enzymes for the photocatalysis of radical reactions opens the door to creating small molecule drugs that cannot be synthesized with traditional chemical approaches.
Summary
Light is widely used in organic synthesis to excite electrons in a substrate or catalyst, opening up reactive pathways to a desired product. Biology uses light sparingly in this way, but coenzymes such as flavin can be driven to excited states by light. A team of researchers investigated this reactivity and found a suite of flavoenzymes that catalyze asymmetric radical cyclization when exposed to light. ''Ene''-reductases, when reduced and illuminated, converted starting materials containing an α-chloroamide and an alkene into five-, six-, seven-, or eight-membered lactams. Different enzymes furnished different stereochemistry in the products, likely because of changes in active-site pocket geometry.
Among the enzymes tested, the flavin-dependent “ene”-reductases from Gluconobacter oxydans (GluER) was effective in the cyclization of α-chloroacetamide to yield γ-lactam when irradiated with near–ultraviolet (UV) light (390 nm). In order to increase the activity of GluER for this non-natural function, the mutation of a residue located at the surface of the protein from threonine to alanine (T36A) resulted in improved yields.
The scientists solved crystal structures of GluER and GluER-T36A using the Frontier Macromolecular Crystallography (FMX) and Automated Macromolecular Crystallography (AMX) beamlines of the National Synchrotron Light Source II (NSLS-II)—a DOE Office of Science User Facility at DOE’s Brookhaven National Laboratory—in order to understand the improved yield of the mutated enzyme. Interestingly, results showed no differences in the structure that would explain the improved yield. Rather, thermodynamics measurements showed that the surface mutation reduced the thermal stability of the protein, thereby altering the protein’s dynamics and improving its function. This mutation did not have a detrimental effect on the native function of the enzyme and allowed the catalyst loading to be decreased to 0.5 mole % without compromising yield or enantioselectivity.
Download the research summary slide
Contact
Todd K. Hyster
Department of Chemistry, Princeton University
thyster@princeton.edu
Publications
K. F. Biegasiewicz, S. J. Cooper, X. Gao, D. G. Oblinsky, J. H. Kim, S. E. Garfinkle, L. A. Joyce, B. A. Sandoval1, G. D. Scholes, T. K. Hyster, “Photoexcitation of flavoenzymes enables a stereoselective radical cyclization.” Science 364, 1166–1169 (2019). DOI: 10.1126/science.aaw1143
Funding
Research reported in this publication was supported by the National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) (R01 GM127703), the Searle Scholars Award (SSP-2017-1741), Sloan Research Fellowship, the Princeton Catalysis Initiative, and Princeton University. D.G.O. acknowledges support from the Postgraduate Scholarships Doctoral Program of the Natural Sciences and Engineering Research Council of Canada. D.G.O. and G.D.S. acknowledge support from the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy (DOE) through grant DE-SC0019370. The AMX (17-ID-1) and FMX (17-ID-2) beamlines of The Life Science Biomedical Technology Research (LSBR) resource is primarily supported by NIH, NIGMS through a Biomedical Technology Research Resource P41 grant (P41GM111244), and by the DOE Office of Biological and Environmental Research (KP1605010). As a National Synchrotron Light Source II facility resource at Brookhaven National Laboratory, work performed at LSBR is supported in part by the DOE Office of Science, Office of Basic Energy Sciences Program under contract DE-SC0012704.
2019-16711 | INT/EXT | Newsroom









