Next Cathode Material: Newly Designed and Freshly Grown
Scientists synthesized a new and promising nickel-rich cathode material with enhanced electrochemical performance
July 31, 2019
 enlarge
enlarge
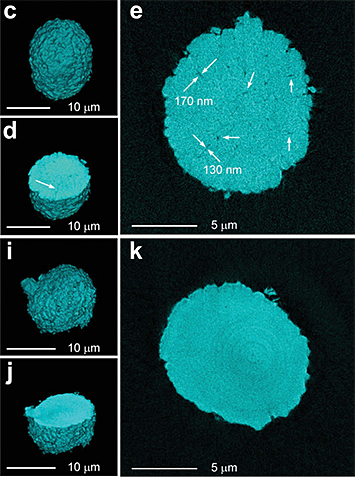
The images above show reconstructions of single particles of the NMC cathode material (NMCs are specific family of cathode materials), while the images to the right show a detailed and enlarged view of a slice of the particle.
The Science
Scientists theoretically designed and successfully synthesized a new titanium (Ti) and lanthanum (La) dual-modified, nickel-rich (Ni-rich) cathode material that shows enhanced electrochemical performance.
The Impact
This new dual-modified route provides a new pathway for commercialization of layered Ni-rich materials for next-generation lithium batteries.
Summary
Lithium-ion batteries (LIBs) provide lightweight, high energy density power sources for a variety of devices, from electric cars to cell phones. Scientists seek new materials to improve the stability and efficiency of these batteries, and layer-structured nickel-rich (Ni-rich) materials are considered as promising cathode candidates for the next-generation of LIBs. This family of cathode materials, which includes lithium (Li), nickel, (Ni), cobalt (Co), and manganese oxide (Mn O) [LiNixCoyMnzO2], is called NMCs. The commercialization of Ni-rich cathodes is still impeded by fast capacity drop and voltage fading due to the interfacial instability and bulk structural degradation of the particles within the cathodes during battery operation.
In this study, scientists theoretically designed and successfully synthesized a new titanium (Ti)-doped and La4NiLiO8-coated LiNi0.8Co0.1Mn0.1O2, a Ni-rich cathode material, which shows enhanced electrochemical performance in terms of rate capability and cycling stability. These kinds of coatings are lanthanum-based oxides and are called LaMO. This improved performance is attributed to the dual approach of Ti doping and La4NiLiO8 coating. The scientists used first principle calculation to direct their theoretical cathode design and then synthesized the new material, adding both modifications in one step. After the synthesis process, the scientists carefully examined the electrochemical performance of the sample. They measured the charge and discharge behavior of the battery during cycling under various voltages and number of cycles. After the electrochemical performance test, the scientists evaluated the structural degradation of the new material. Among these other methods, they used transmission x-ray microscopy at the Full Field X-ray Imaging (FXI) beamline at the National Synchrotron Light Source II (NSLS-II)—a U.S. Department of Energy (DOE) Office of Science User Facility at DOE’s Brookhaven National Laboratory—to capture of the morphology and chemical state evolution of the material at the nanoscale.
The scientists concluded that the proposed strategy of a dual-modified route provides a rational approach for the development of an advanced cathode material for high-performance lithium-ion batteries, and they assume that the approach could also be extended to other high capacity cathode materials to achieve enhanced performance.
Download the research summary slide
Contact
L. J. Li
University of Science and Technology Changsha, China
lingjun.li@csust.edu.cn
Q. B. Zhang
University Xiamen, Fujian, China
zhangqiaobao@xmu.edu.cn
J. Lu
Argonne National Laboratory, Argonne, USA
junlu@anl.gov
Publications
H. Yang, “Simultaneously Dual Modification of Ni-Rich Layered Oxide Cathode for High-Energy Lithium-Ion Batteries”, Adv. Funct. Mater. 29, 1808825 (2019). DOI: 10.1002/adfm.201808825
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51774051, 51304031, and 21703185), National Key R&D Program of China (Grant No. 2018YFB0905400). This work was also supported by the Changsha City Fund for Distinguished and Innovative Young Scholars (Grant No. KQ1707014) and the Hunan Provincial Natural Science Foundation of China (Grant No. 2018JJ2428). Additional support was provided from the U. S. Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office. Argonne National Laboratory is operated for DOE Office of Science by UChicago Argonne, LLC, under Contract No. DE-AC02-06CH11357. This research used resources at FXI beamline (18-ID) of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-AC02-98CH10886.
2019-16768 | INT/EXT | Newsroom









