Kinase Activation Mechanism Could Help Cancer Research
Scientists investigate the activation mechanism of Akt 1 protein kinase to understand its role in cancer proliferation
January 31, 2019
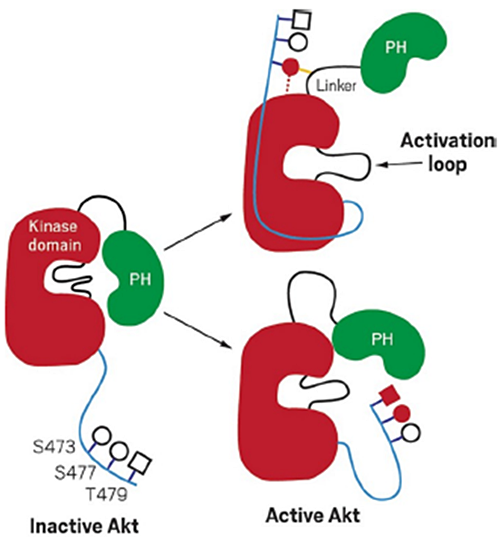
Inactive Akt1 can be activated by two mechanisms. In one mechanism, phosphorylated serine 473 interacts with the linker between the kinase and PH domain. In the other, phosphorylated serinve 477 and Threonine 479 result in the displacement of the PH domain. Image courtesy of Cell 174(4):897-907 (2018).
The Science
Scientists revealed the activation mechanism of Akt1 protein kinase, which modulates metabolism and cancer proliferation.
The Impact
Akt 1 protein kinase plays an essential role in cancer proliferation, which defines cancer aggressiveness. Understanding the full functions of the protein could help advance cancer therapies.
Summary
Scientists revealed a different activation mechanism of Akt1 protein kinase by closely studying its molecular interactions.
Akt1 is a critical protein kinase that drives cancer proliferation, modulates metabolism, and is activated by C-terminal phosphorylation. Phosphorylation is an important cell mechanism that manages the activation of proteins by adding a phosphate group to the protein. Just like computer programs, proteins have a specific job in a cell and they can be signaled to start and stop working.
To better understand how proteins function within cells, scientists studied their three-dimensional structure and built models. The current structural model for Akt1 activation is centered on intramolecular interactions between the C-terminal tail and a certain region, called N lobe, of the kinase domain. In this study, the scientists used a site-specifically phosphorylated form of Akt1 that is well suited for mechanistic analysis.
By using biochemical, x-ray crystallographic, and cellular approaches, they determined that the activation is driven by an intramolecular interaction between the C-tail and a different domain of the protein called pleckstrin homology (PH)-kinase domain linker. The scientists used the Highly Automated Macromolecular Crystallography (AMX) beamline and Frontier Microfocusing Macromolecular Crystallography (FMX) beamline to measure the crystalized protein structure at the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory.
Their results provide a new framework for understanding how Akt 1 is controlled in cell signaling and suggest distinct functions for differentially modified Akt forms. Understanding the full functions of the protein could help advance cancer therapies.
Download the research summary slide
Contact
Sandra Beatriz Gabelli
Johns Hopkins School of Medicine
gabelli@jhmi.edu
Philip Arthur Cole
Harvard Medical School and Johns Hopkins School of Medicine
pacole@bwh.harvard.edu
Publications
N. Chu, A. L. Salguero, A. Z. Liu, Z. Chen, D. R. Dempsey, S. B. Ficarro, W. M. Alexander, J. A. Marto, Y. Li, L. M. Amzel, S. B. Gabelli, P. A. Cole. “Akt Kinase Activation Mechanisms Revealed Using Protein Semisynthesis”, Cell 174(4):897-907 (2018). DOI: 10.1016/j.cell.2018.07.003
Funding
This work was funded by NIH (CA74305 and CA062924) and FAMRI Foundation. This research used beamlines 17-ID-1 and 17-ID-2 of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. The Life Science Biomedical Technology Research resource (LSBR) at NSLS-II is supported by NIGMS grant P41GM111244 and US Department of Energy grant DOE KP1605010. This research used resources of the Advanced Photon Source, a facility operated for the DOE Office of Science by Argonne National Laboratory under contract DE-AC02-06CH11357. Use of the beamline at Sector 31 of the Advanced Photon Source was provided by the Eli Lilly Company.
2019-17265 | INT/EXT | Newsroom