New Insights into the Synthesis Process of Layered Oxides
Scientists investigated the synthesis of high-performance layered oxide cathode materials for next-generation batteries
September 30, 2018
 enlarge
enlarge
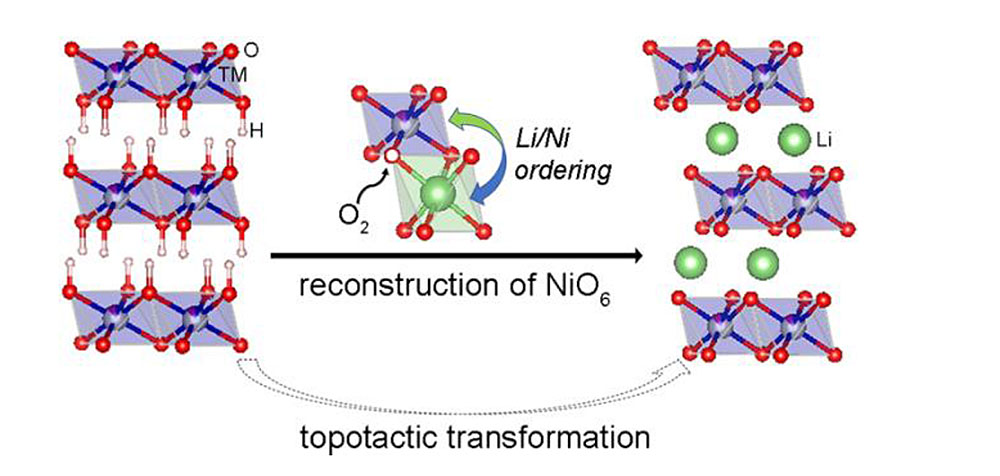
By using a combination of in situ x-ray characterization techniques at the SRX and XPD beamlines at NSLS-II, scientists investigated the structural evolution of a transition metal layered oxide during its synthesis. New insights from this study improved the understanding of how the layers arrange on larger and smaller scales. Image credit: J. Am. Chem. Soc., 140, 39, 12484-12492 (2018)
The Science
Scientists discovered that the synthesis reaction pathway of layered transition metal oxides is dominated by symmetry breaking and reconstructing basic building units (BBUs), consequently affecting the performance of these materials as battery cathodes.
The Impact
Understanding how these materials grow may provide general principles for the design and synthesis of high-performance cathodes for commercial lithium-ion (Li-ion) batteries.
Summary
Many of the devices we use in our daily lives, such as smartphones and laptops, rely on batteries and microelectronics that are made from materials like metal oxides. These materials are particularly interesting to scientists because many of their properties can be directly connected to their atomic structure, or their basic building units (BBUs), and how they are ordered.
One example is high-nickel (high-Ni) transition metal layered oxides, which are intensely pursued worldwide for potential use in Li-ion batteries. Even though it is known that the electrochemical properties of these materials come from the ordering of their BBUs, it is still unknown how this ordering is achieved during the synthesis process of the material.
In this study, scientists used a multimodal in situ x-ray characterization approach to investigate the synthesis process of a specific high-Ni transition metal layered oxide, LiNi0.77Mn0.13Co0.10O2. The scientists investigated the ordering of the material on different length scales, from the long range to local individual units—the BBUS.
To track the structural evolution in these length scales, the scientists used a combination of x-ray techniques at the National Synchrotron Light Source II (NSLS-II) and the Cornell High Energy Synchrotron Source. NSLS-II is a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory.
At NSLS-II, the team worked with scientists from the Submicron Resolution X-ray Spectroscopy (SRX) beamline and the X-ray Powder Diffraction (XPD) beamline to show how defects in the structure affect the performance of these materials as battery cathodes.
In their real-time observations supported by first-principles calculations the scientists learned that the oxidation of cobalt (Co) and manganese (Mn) is preferred over Ni oxidation, which lead to significant changes in the local structure, including oxygen (O) loss and the breaking of symmetry. The consequence of this is that Ni2+ ions become highly mobile and tend to mix with Li, causing disordering upon formation of the layered oxides. The scientists also learned that only through high-temperature heat treatment, the Ni is further oxidized and induces symmetry reconstruction.
The findings shed light on synthesis of high-Ni layered oxide cathodes by design and, more broadly, various functional materials through synthetic control of the constituent BBUs.
Download the research summary slide
Contact
Feng Wang
Brookhaven National Laboratory
fwang@bnl.gov
Feng Pan
Peking University
panfeng@pkusz.edu.cn
Khalil Amine
Argonne National Laboratory
amine@anl.gov
Jianming Bai
Brookhaven National Laboratory
jmbai@bnl.gov
Publication
M. Zhang, G. Teng, Y. K. Chen-Wiegart, Y. Duan, J. Young, P. Ko, J. Zheng, J. Thieme, E. Dooryhee, Z. Chen, J. Bai, K. Amine, F. Pan, F. Wang. Cationic Ordering Coupled to Reconstruction of Basic Building Units during Synthesis of High-Ni Layered Oxides. J. Am. Chem. Soc., 140, 39, 12484-12492 (2018). DOI: 10.1021/jacs.8b06150
Funding
This work was supported by the U.S. Department of Energy (DOE) Office of Energy Efficiency and Renewable Energy under the Advanced Battery Materials Research (BMR) program, Contract No. DE-SC0012704, the National Materials Genome Project (2016YFB0700600), and the Shenzhen Science and Technology Research Grants (JCYJ20150729111733470, JCYJ20151015162256516). Research at Argonne National Laboratory is supported by the DOE, Vehicle Technologies Office, and the US-China Clean Energy Research Center – Clean Vehicle Consortium (CERC-CVC). Argonne National Laboratory is operated for DOE Office of Science by UChicago Argonne, LLC, under contract number DE-AC02-06CH11357. This research used the 5-ID (SRX), 28-ID-2 (XPD) beamlines of the National Synchrotron Light Source II, a DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. SEM/EDX and TEM measurements carried out at the Center for Functional Nanomaterials, Brookhaven National Laboratory, were supported by the DOE, Office of Basic Energy Sciences, under Contract No. DE-SC0012704. The synchrotron XRD work conducted at the Cornell High Energy Synchrotron Source was supported by the National Science Foundation under award DMR-1332208.
2018-17585 | INT/EXT | Newsroom









