Understanding Recycling in Cells
Scientists uncovered an elusive structure and function of a protein complex involved in autophagy
January 31, 2018
 enlarge
enlarge
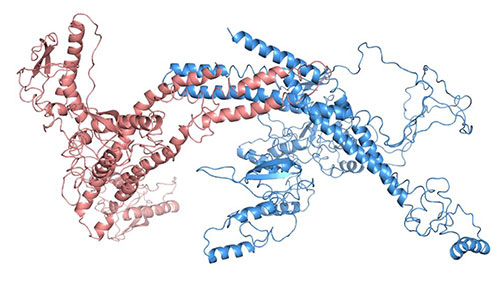
The image shows a structural model of the Snx4 (red) and Atg20 (blue) complex, which has been very challenging to investigate using the current structural methods. Scientists studied this complex using SAXS at the LiX beamline at NSLS-II to provide the first insight into the architecture of this important protein complex. Image credit: PNAS, 2017, E10112–E10121
The Science
Small-angle x-ray Scattering with in-line size exclusion chromatography helped scientists develop a new structure-function model for the Snx4-Atg20 complex in cellular autophagy.
The Impact
Autophagy is a cellular process that ensures damaged or long-lived cellular components are recycled to prevent damage to the cell. Defects in autophagy have been correlated with cancer and neurodegenerative diseases.
Summary
Diseases, such as cancer and neurodegenerative illnesses, are linked to malfunctioning processes in cells. One of these processes is an essential pathway called macroautophagy/autophagy. Autophagy is a form of cellular self-cleaning, where cytoplasmic material destined for degradation is captured in double-membrane compartments that mature and fuse with a degradative organelle. Highly dynamic proteins engaged in autophagy are very challenging to study using standard techniques that reveal a 3D structure.
The Atg1 kinase complex initiates autophagy in yeast upon nitrogen starvation, but one member of this complex, the sorting nexin Atg20, has remained relatively uncharacterized.
In this study, scientists unlocked, for the first time, the function and structure of Atg20 in a dimer with Snx4/Atg24, another sorting nexin that is a component of the kinase complex in yeast. The researchers worked with scientists from the Life Science X-ray Scattering (LiX) beamline to map the structure of the Atg20-Snx4 heterodimer using a combination of computational modeling and small-angle x-ray scattering (SAXS). The LiX beamline is part of the structural biology research suite at the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory. LiX is dedicated to investigating complex structures, and offers short measurement times due to its high throughput capabilities.
This investigation shed light on the overall shape of the Atg20-Snx4 heterodimer that is required for efficient autophagy and membrane tubulation.
Download the research summary slide
Contact
Daniel J. Klionshy
University of Michigan
klionsky@umich.edu
Michael J. Ragusa
Dartmouth College
Michael.j.ragusa@dartmouth.edu
Publication
H. Popelka, A. Damasio, J. E. Hinshaw, D. J. Klionsky, and M. J. Ragusa. Structure and function of yeast Atg20, a sorting nexin that facilitates autophagy induction. PNAS, 2017, E10112–E10121. DOI: 10.1073/pnas.1708367114
Funding
This work was supported by National Institutes of Health Grant GM053396 (to D.J.K.) and the Protein Folding Diseases FastForward Initiative, University of Michigan. M.J.R. is supported by National Institutes of Health Grant GM113132. J.E.H. is supported by National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program. SAXS data were collected at the life science X-ray scattering (LiX) beamline at National Synchrotron Light Source II (NSLS-II). LiX operates under Department of Energy (DOE) Biological and Environmental Research Contract DE-SC0012704 and is supported by National Institutes of Health–National Institute of General Medical Sciences Grant P41GM111244. NSLS-II is operated under DOE Basic Energy Sciences Contract DE-SC0012704.
2018-18959 | INT/EXT | Newsroom









