Combining X-rays & Neutrons to Study Molten Salts
A team of scientists discovered an unexpected lower melting temperature in molten salts
December 31, 2021
 enlarge
enlarge
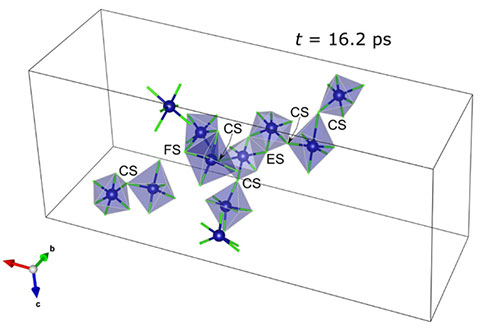
The schematic depicts simulated structures showing CrCl6 octahedra molecular chains that compose the NaCl-CrCl3 molten salt (EC: edge shared, FC: face shared, and CS: corner shared). These chains were also observed in the experimental measurements. Image credit: Energy Mater., 11, 2003190 (2021)
The Science
Scientists determined the atomic structure of chromium (Cr)-containing molten salts & discovered a much lower melting temperature than expected, and a broad metastable liquid−solid coexistence phase.
The Impact
Cr is the principal corrosion product in molten salt nuclear reactors and solar energy installations. The arrangement of ions around Cr must be determined to predict changes in molten salt properties due to corrosion.
Summary
With the ever-increasing demand for energy, researchers are working on safer and more efficient nuclear reactor designs. One potential candidate is molten salt reactors (MSRs). These reactors use molten salt mixtures as either coolants or fuel or both. To make MSRs a reality, researchers need to understand the complex chemical interplay between the molten salts, reactor fuels, and potential impurities. One major factor in this interplay is corrosion, which changes the composition and physical properties of salts during operations.
In this study, a team of researchers studied the atomic structure of molten NaCl−CrCl3 because chromium (Cr) is the principal corrosion product. The team found networks of molecular chains made of CrCl6 octahedra and determined temperature-dependent changes in the atomic structure. These findings agreed remarkably well with their ab initio molecular dynamic simulations.
For their measurements, the team used neutron scattering at the Spallation Neutron Source (SNS) and x-ray scattering measurements at the Pair Distribution Function (PDF) beamline at the National Synchrotron Light Source II (NSLS-II). The PDF beamline is part of a suite of advanced x-ray tools to investigate the atomic structure and chemical dynamics of materials at NSLS-II. NSLS-II and SNS are U.S. Department of Energy (DOE) Office of Science User Facilities located at Brookhaven National Laboratory and Oak Ridge National Laboratory, respectively.
The results from this study could be utilized as experimental validation for developing novel atomistic models, such as neural network interatomic potentials. These models will help researchers predict the behavior of molten salts in operating nuclear reactors.
Download the research summary slide
Contact
Ju Li
Massachusetts Institute of Technology
liju@mit.edu
Boris Khaykovich
Massachusetts Institute of Technology
bkh@mit.edu
Publications
Q.-J. Li, D.J. Sprouster, G. Zheng, J. Neuefeind, A. Braatz, J. Mcfarlane, D. Olds, S. Lam, J. Li, and B. Khaykovich, Complex Structure of Molten NaCl-CrCl3 Salt: Cr-Ion Network and Intermediate-Range Order, ACS Applied Energy Materials, 4, 4, 3044-3056, 2021; DOI: 10.1021/acsaem.0c02678
Funding
We acknowledge multiple useful discussions with Richard Mayes, Stephen Raiman, Jake McMurray (ORNL), and Raluca Scarlat (UC Berkeley). We acknowledge the help of Abbey McAllister (ORNL) with sample handling and preparation. This material is based upon work supported by the Department of Energy under Award Number DENE0008751. This research used resources at the Spallation Neutron Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated by the Oak Ridge National Laboratory, and the beamline 28-ID-1 (PDF) of the National Synchrotron Light Source II, a DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. This work also used the Extreme Science and Engineering Discovery Environment (XSEDE) resources at the Texas Advanced Computing Center (TACC) through allocation DMR190055.
2021-19509 | INT/EXT | Newsroom









