A Better Catalyst for Propylene Oxide Production
June 14, 2024
 enlarge
enlarge
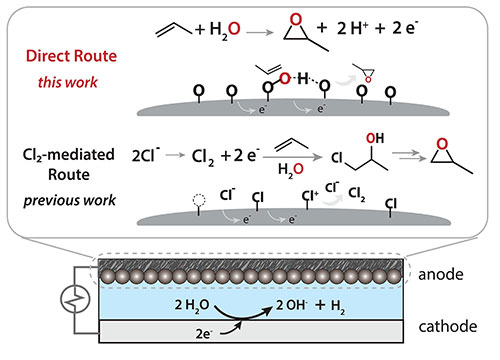
Comparison of the catalysis approach described in this work and one of the alternative approaches, which is chlorine-mediated. Credit: Science 383 6678, pp 49-55 (2024)
The Science
Researchers identified and characterized a catalyst designed to more safely yield the industrial chemical propylene oxide.
The Impact
Avoiding the dangerous compounds involved in the catalysis of propylene oxide—widely used to create plastics—would greatly reduce the associated environmental hazards.
Summary
A collaboration of scientists from the California Institute of Technology and the Massachusetts Institute of Technology have found a catalyst that may greatly reduce the hazards and environmental impacts of the production of a widely used industrial chemical, propylene oxide.
Propylene oxide has multiple uses and plays a key role in the manufacture of a variety of products, including a broad class of plastics. But the typical approaches to producing it either require the use of dangerous compounds or yield toxic side products. This new catalyst, on the other hand, makes use of the oxygen atom found in the water molecule and has a side product of hydrogen gas, which could be collected to use as fuel or to make other products.
The catalyst is a combination of platinum oxide and palladium oxide. These two compounds are, separately, capable of catalyzing the reaction, but neither does a stellar job. However, when the platinum oxide is embedded into a palladium oxide crystal structure, the benefits of both catalysts are amplified.
The group studied the catalyst, in part, at the U.S. Department of Energy’s (DOE) National Synchrotron Light Source II, a DOE Office of Science User Facility located at Brookhaven National Laboratory. At NSLS-II's Inner-Shell Spectroscopy (ISS) beamline, the group used x-ray absorption spectroscopy to examine the catalyst’s structure and electronic behavior. They found that the success of the catalyst comes from the platinum oxide: The oxygen atom attached to the platinum becomes electron-depleted, thereby “wanting” to react with the electron-rich propylene.
In future work, the group will continue testing the catalyst to determine how it could transition from a small-scale research setting to actual industrial use.
Download the research summary slide (PDF)
Contact
Karthish Manthiram
California Institute of Technology
karthish@caltech.edu
Publications
Chung, M., Maalouf, J.H., Adams, J.S., Jiang, C., Román-Leshkov, Y., Manthiram, K. "Direct propylene epoxidation via water activation over Pd-Pt electrocatalysts." Science Vol 383, Issue 6678 pp. 49-55, DOI: 10.1126/science.adh4355
Funding
This research was supported by the US Department of Energy (DOE) Office of Science, Office of Basic Energy Sciences, Catalysis Science Program, award no. DE-SC0023207. K.M. gratefully acknowledges the support of the Sloan Foundation. This research used resources of the National Synchrotron Light Source II, a US DOE Office of Science User Facility operated by Brookhaven National Laboratory under contract no. DE-SC0012704.
2024-21949 | INT/EXT | Newsroom