An AI-Based Biomolecular Structure Prediction & Design Tool
September 30, 2024
The Science
Researchers developed a protein structure prediction & design tool, and experimentally validated its designs.
The Impact
This capability could significantly impact drug design, biotechnology, and the study of biological processes.
Summary
A collaboration of researchers from the University of Washington, Sheffield University (UK), and Seoul National University (Korea) have developed a next-generation artificial intelligence (AI)-based tool for predicting and designing the structures of proteins and biomolecular assemblies. They were also able to experimentally validate the success of some of those designs using, in part, the Highly Automated Macromolecular Crystallography (AMX) beamline at National Synchrotron Light Source II, a U.S. Department of Energy (DOE) user facility located at DOE’s Brookhaven National Laboratory.
The work is a significant step for protein structure prediction, allowing for modeling of general biomolecular assemblies in “real world” ways — proteins in complex with other proteins, proteins interacting with DNA and RNA, etc. This has been a challenge: While deep learning methods have revolutionized protein structure prediction, they have only been capable of yielding protein-only systems. This tool, known as RoseTTAFold All-Atom (RFAA), can predict assemblies containing proteins, nucleic acids, small molecules, metals, and chemical modifications. It should prove to be widely useful in the fields of drug design, biotechnology, and biomedical research, and in the study of biological processes.
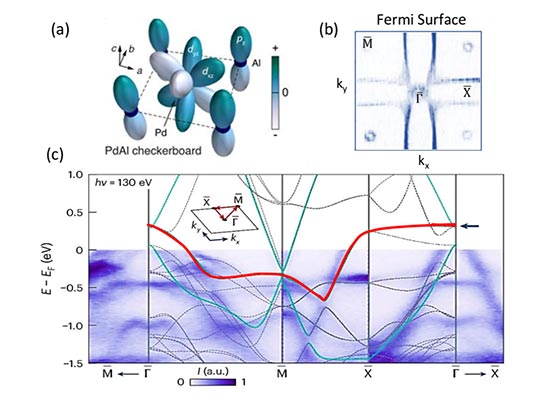
RFAA is based upon an existing neural network, RoseTTAFold2, which was developed by a group led by one of the paper’s authors, David Baker, the director of the University of Washington’s Institute of Protein Design. RoseTTAFold2 takes inputs as one-dimensional (1D), 2D, and 3D “tracks.” Here, the 1D track was inputed with the chemical element type of each nonpolymer atom, the 2D track was for the chemical bonds between atoms, and the 3D track took information on chirality, a geometric characteristic that defines whether a molecule can be superimposed onto its mirror image (also sometimes called “handedness”).
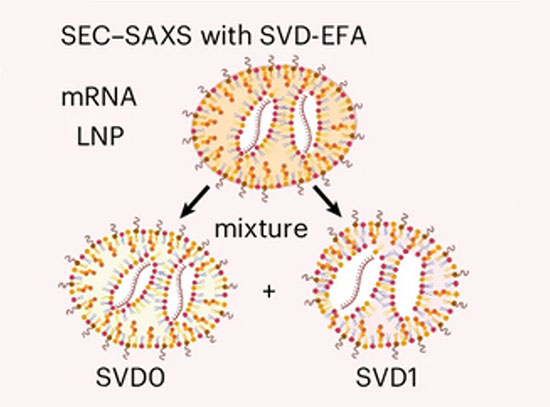
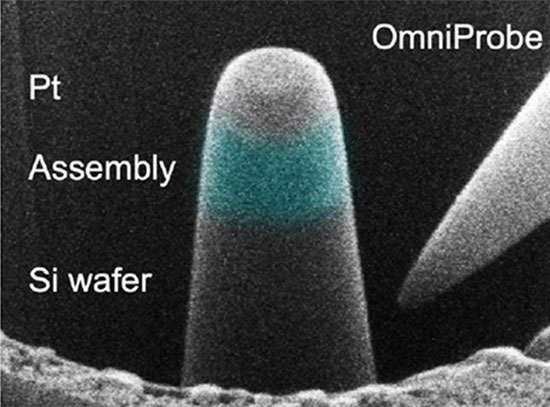
The group also developed a biomolecular design tool, RFdiffusion All-Atom. It generates folded protein structures that interact with non-protein biomolecules, including DNA, RNA, and small molecules. The team attempted to create novel proteins that would bind target small molecules, including the cardiac disease drug digoxigenin. In each case, experimental characterization showed that a subset of the generated proteins did have the desired binding activity. This demonstrates that protein:small molecule binding can now be generated using AI.
Download the research summary slide (PDF)
Contact
David Baker
University of Washington
dabaker@uw.edu
Publications
Rohith Krishna, Jue Wang, Woody Ahern, Pascal Sturmfels, Preetham Venkatesh, Indrek Kalvet, Gyu Rie Lee, Felix S. Morey-Burrows, Ivan Anishchenko, Ian R. Humphreys, Ryan McHugh, Dionne Vafeados, Xinting Li, George A. Sutherland, Andrew Hitchcock, C. Neil Hunter, Alex Kang, Evans Brackenbrough, Asim K. Bera, Minkyung Baek, Frank DiMaio, and David Baker. Generalized biomolecular modeling and design with RoseTTAFold All-Atom, Science, 384 (6693) (2024). DOI: https://www.science.org/doi/10.1126/science.adl2528
Funding
We thank Microsoft for their generous donation of Azure Compute Credits, and Perlmutter grant NERSC award BER-ERCAP0022018 for access to the Perlmutter high-performance computing resources. This work was supported by gifts from Microsoft (R.K., P.S., D.B.); the Howard Hughes Medical Institute (D.B., G.R.L.); the New Faculty Startup Fund from Seoul National University (M.B.); the Schmidt Futures program (J.W., R.M., F.D.); the Open Philanthropy Project Improving Protein Design Fund (J.W., I.K., G.R.L.); grant no. INV-010680 from the Bill and Melinda Gates Foundation (W.A.); the Audacious Project (P.V.); the Washington State General Operating Fund supporting the Institute for Protein Design (P.V.); the Defense Threat Reduction Agency (DTRA) (G.R.L.); the Washington Research Foundation’s Innovation Fellows Program (G.R.L.); a Faculty of Science PhD studentship from the University of Sheffield (F.S.M.-B.); federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. 75N93022C00036 (I.A.); Amgen (I.R.H.); Juvenile Diabetes Research Foundation International (JDRF) grant no. 2-SRA-2018-605-Q-R (X.L.); Helmsley Charitable Trust Type 1 Diabetes (T1D) Program grant no. 2019PG-T1D026 (X.L.); Defense Threat Reduction Agency grant HDTRA1-19-1-0003 (X.L.); Bill and Melinda Gates Foundation no. OPP1156262 (X.L.); Synergy award 854126 from the European Research Council (G.A.S., C.N.H.); a Royal Society University Research Fellowship (award no. URF\R1\191548 to A.H.); and Human Frontiers Science Program grant RGP0061/2019 (F.D.).
2024-22225 | INT/EXT | Newsroom