Fighting Cancer with DNA Origami
innovation at CFN
March 30, 2020
 enlarge
enlarge
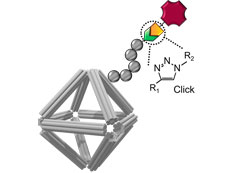
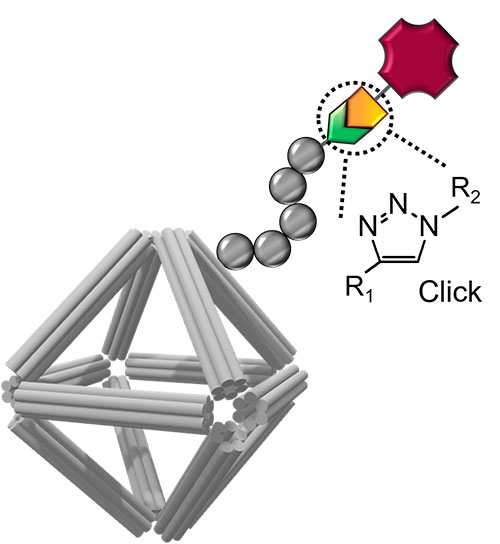
Schematic of a DNA origami stabilized by peptoid coating and equipped with imaging and cell-targeting capabilities. Click chemistry was used to conjugate functional molecules on the peptoid/origami surface.
What is the scientific achievement?
CFN scientists and a team of collaborators have devised a new approach for building nanomaterials for use as nanomedicines. The team developed a class of biocompatible molecular coatings and used them to stabilize wireframed DNA origami cages. The coatings give the structure multifunctionality and environmental stability. In this work, the researchers showed that the designed nanomaterials are capable of carrying an anticancer drug and delivering medicines with a controllable release.
Why does this achievement matter?
Although DNA nanotechnology provides a toolkit for creating programmable nanostructures with potential for biomedical applications, a challenge is the limited structural integrity of these materials in complex biological fluids. The molecular coatings developed in this work solve this challenge, paving the way for this approach to be used in drug delivery, bioimaging, and cellular targeting.
What are the details?
DNA nanotechnology has established approaches for designing programmable and precisely controlled nanoscale architectures through specific Watson−Crick base-pairing, molecular plasticity, and intermolecular connectivity. In particular, superior control over DNA origami structures could be beneficial for biomedical applications, including biosensing, in vivo imaging, and drug and gene delivery. However, protecting DNA origami structures in complex biological fluids while preserving their structural characteristics remains a major challenge for enabling these applications. Here, the authors developed a class of structurally well-defined peptoids to protect DNA origamis in ionic and bioactive conditions and systematically explored the effects of peptoid architecture and sequence dependency on DNA origami stability. The applicability of this approach for drug delivery, bioimaging, and cell targeting was also demonstrated. Nine peptoids with two types of architectures, termed as “brush” and “block,” were built from positively charged monomers and neutral oligo-ethyleneoxy monomers, where certain designs were found to greatly enhance the stability of DNA origami. Through experimental and molecular dynamics studies, the role of sequence-dependent electrostatic interactions of peptoids with the DNA backbone was demonstrated. The authors showed that octahedral DNA origamis coated with peptoid (PE2) can be used as carriers for anticancer drug and protein, where the peptoid modulated the rate of drug release and prolonged protein stability against proteolytic hydrolysis. Finally, two alkyne-modified peptoids were synthesized and conjugated with fluorophore and antibody, to make stable DNA origamis with imaging and cell-targeting capabilities. The results demonstrate an approach toward functional and physiologically stable DNA origami for biomedical applications.
CFN Capabilities
CFN Materials Synthesis and Characterization facilities were used to create the DNA origamis, and the CFN Electron Microscopy Facilities and NSLS-II were used for characterization. Peptoid coatings were synthesized at the Molecular Foundry at LBL.
Publication Reference
S.–T. Wang, S. Xuan, M.A. Gray, Y. Lin, A.I. Nguyen, N. Todorova, M.M. Stevens, C.R. Bertozzi, R.N. Zuckermann, O. Gang. DNA Origami Protection and Molecular Interfacing through Engineered Sequence-Defined Peptoids, Proceedings of the National Academy of Sciences of the United States of America 117, 6339 (2020).
DOI: 10.1073/pnas.1919749117; www.pnas.org/cgi/doi/10.1073/pnas.1919749117
U.S. Provisional Patent Application: 62/897,451 “Side Chain Modified Peptoids Useful as Structure-Stabilizing Coating for Biomaterials”.
PNAS In This Issue: https://www.pnas.org/content/117/12/6279
BNL newsroom: https://www.bnl.gov/newsroom/news.php?a=117042
Molecular Foundry (LBNL) news: https://foundry.lbl.gov/2020/03/09/peptoid-dna-origami/
Stanford ChEM-H news:
https://twitter.com/Stanford_ChEMH/status/1239551999609200641?s=20
https://chemh.stanford.edu/news/protecting-dna-origami-anti-cancer-drug-delivery
Acknowledgement of Support
This work was supported by the Center for Functional Nanomaterials, the Molecular Foundry, the Laboratory Directed Research and Development grant, and the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy (DOE) under Contracts DE-SC0012704 and DE-AC02-05CH11231. The DNA origami work was supported by the US DOE, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, under Grant DE-SC0008772. The LiX beamline is part of the Life Science Biomedical Technology Research resource, cofunded by National Institute of General Medical Sciences Grant P41 GM111244 and by DOE Office of Biological and Environmental Research Grant KP1605010, with additional support from NIH Grant S10 OD012331. The operation of National Synchrotron Light Source II is supported by US DOE, Office of Basic Energy Sciences Contract DE-SC0012704. MD simulations were supported by computational resources provided by the Australian Government through National Computational Infrastructure Project e90 under the National Computational Merit Allocation Scheme. We thank Dr. David Rabuka (Catalent) for generously providing fGly-modified antibody and the Stanford University Mass Spectrometry facility and Theresa McLaughlin for performing characterization of the intact proteins. Y.L. and M.M.S. acknowledge support from the European Research Council Seventh Framework Programme Consolidator Grant “Naturale CG” (616417).
2020-17197 | INT/EXT | Newsroom