- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Electrochemical Energy Storage
Tuning Charge-discharge Induced Unit-cell-breathing through Metal-Metal Bonding in Layer-structured Cathode Materials for Lithium-ion Batteries
In collaboration with the Institute of Physics, Chinese Academy of Sciences, we have conducted a systematic study of lithium molybdenum trioxide (Li2MoO3). A a new “unit-cell-breathing” mechanism is introduced based on both crystal and electronic structural changes of transition metal oxide cathode materials during charge-discharge: for widely used LiMO2 (M= Co, Ni, Mn), lattice parameters a and b contract during charge. However, for Li2MoO3, such changes are in opposite directions. Metal-metal bonding is used to explain such “abnormal” behavior and a generalized hypothesis is developed. The expansion of the M-M bond becomes the controlling factor for a(b) evolution during charge, in contrast to the shrinking M-O bond as a controlling factor in “normal” materials. The cation mixing caused by migration of Mo ions at higher oxidation state provides the benefits of reducing the c expansion range at an early stage of charging and suppressing the structure collapse at high voltage charge. These results open up a new strategy for designing and engineering layered cathode materials for high-energy-density lithium-ion batteries.
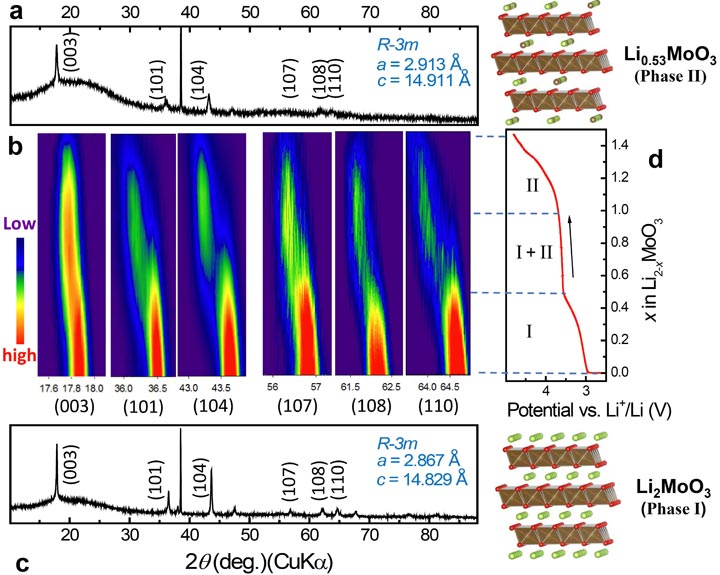
“Tuning charge-discharge induced unit-cell-breathing through metal-metal bonding in layer-structured cathode materials for lithium-ion batteries,” Y. Zhou, J. Ma, E. Hu, X. Yu, L. Gu, K.W. Nam, L. Chen, Z.X. Wang, X.-Q. Yang, Nature Commun. (2014), 5, 6381.




