- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Catalysis: Reactivity and Structure
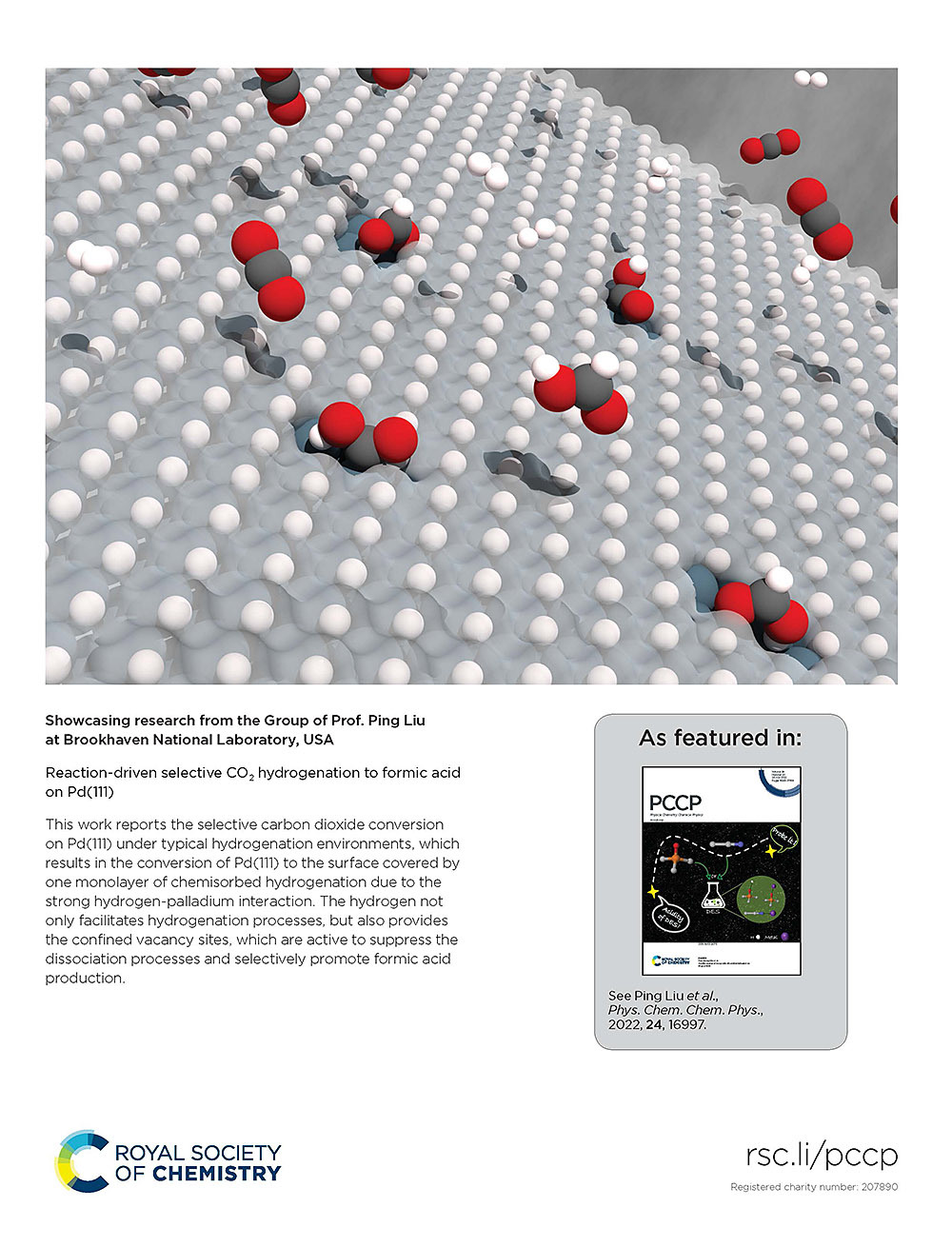
“Reaction-Driven Selective CO2 Hydrogenation to Formic Acid on Pd(111)”, Physical Chemistry Chemical Physics 24 (2022) 16997-17003.
This work reports the selective carbon dioxide conversion on Pd(111) under typical hydrogenation environments, which results in the conversion of Pd(111) to the surface covered by one monolayer of chemisorbed hydrogenation due to the strong hydrogen-palladium interaction. The hydrogen not only facilitates hydrogenation processes, but also provides the confined vacancy sites, which are active to suppress the dissociation processes and selectively promote formic acid production.

“Identification of Highly Selective Surface Pathways for Methane Dry Reforming using Mechano-Chemical Synthesis of Pd-CeO2”, ACS Catalysis 12 (2022) 12809–12822.
Pd entities on ceria generated by mechanochemical milling are able to tune DRM selectivity by easier hydrogen and/or carbon doping into the metal lattice.

“Potassium-Promoted Methanol Synthesis from CO2 Hydrogenation over CuxO/Cu(111) (x≤2) Model Surface: Rationalizing the Potential of Potassium in Catalysis” ACS Catalysis 10 (2020) 5383-5958.
Potassium was found to act as an active center for selective tuning in binding, an accelerator for charge transfer, and a mediator for the electron tunneling, being able to facilitate the selective conversion of carbon dioxide to methanol on CuxO/Cu(111).

“Cesium-Induced Active Sites for C–C Coupling and Ethanol Synthesis from CO2 Hydrogenation on Cu/ZnO(0001̅) Surfaces”, Journal of the American Chemical Society 143 (2021) 13103–13112.
The deposition of Cs on Cu/ZnO(0001̅) is found to greatly facilitate methanol synthesis and enable the ethanol synthesis from CO2 hydrogenation. The synergy among Cs, Cu, and ZnO at the interface plays an essential role, being able to promote the CO2 and tune the selectivity toward methanol and ethanol.
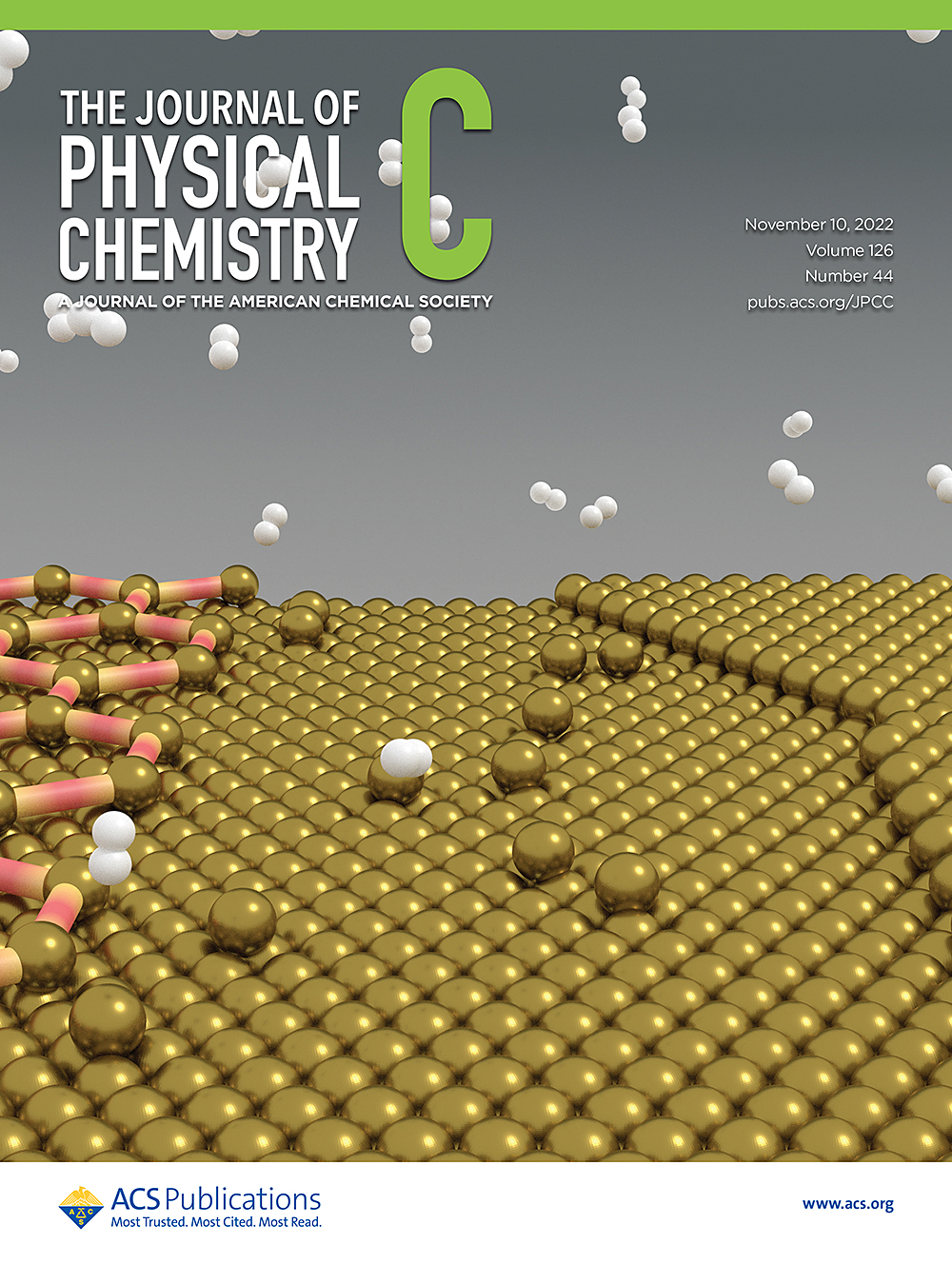
“Enhanced Oxide Reduction by Hydrogen at Cuprous Oxide-Copper Interfaces near Ascending Step Edges”, The Journal of Physical Chemistry C 126 (2022) 18645-18651.
Reduction of Cu2O/Cu(111) by H2 is facilitated by nearby ascending step edges on the metallic substrate. Free copper atoms flowing from step edges create dynamic local ensembles that facilitate the activation of molecular hydrogen.

“Highly Selective Methane to Methanol Conversion on SnO2/Cu2O/Cu(111) Catalysts: Unique Properties of SnO2 Nanostructures and the Inhibition of the Direct Oxidative Combustion of Methane”, ACS Catalysis 12 (2022) 11253–11262.
Inverse catalysts generally consist of oxide nanoparticles supported on metal substrates, which can exhibit exceptional catalytic properties. The small SnO2 nanoparticles uniformly dispersed on a Cu2O/Cu(111) substrate enabled a unique SnO2–Cu2O interface that can completely convert methane to methanol directly under the environments of oxygen and water.

“Low Temperature Activation of Methane on Metal-Oxides and Complex Interfaces: Insights from Surface Science”, Accounts of Chemical Research 53 (2020) 1488–1497.
New materials for the efficient C–H activation of methane at low temperature has opened interesting possibilities for the specific production of chemicals such as methanol directly from methane, a step toward facile synthesis of liquid fuels.

“Establishing structure-sensitivity of ceria reducibility: real-time observations of surface-hydrogen interactions”, Journal of Materials Chemistry A 8 (2020) 5501-5507.
The effect of the last layer of atoms on surface reduction is established using a unique combination of morphological, structural, and chemical analyses of a model ceria catalyst with different surface terminations under an H2 environment.

“Anion-mediated electronic effects in reducible oxides: Tuning the valence band of ceria via fluorine doping”, Journal of Chemical Physics 151 (2019) 044701.
Incorporation of fluoride ions into a prototypical reducible oxide surface, namely, ceria(111), can induce a variety of nontrivial changes to the local electronic structure, beyond the expected increase in the number of Ce3+ ions.




