- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Catalysis: Reactivity and Structure
A New Type of Strong Metal-Support Interaction and the Production of H-2 through the Transformation of Water on Pt/CeO2(111) and Pt/CeOx/TiO2(110) Catalysts
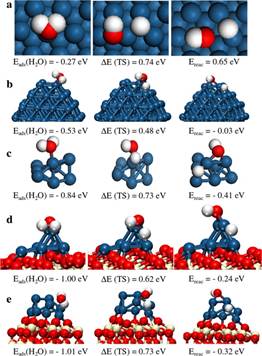
The electronic properties of Pt nanoparticles deposited on CeO2(111) and CeOx/TiO2(110) model catalysts have been examined using valence photoemission experiments and density functional theory (DFT) calculations. The valence photoemission and DFT results point to a new type of “strong metal–support interaction” that produces large electronic perturbations for small Pt particles in contact with ceria and significantly enhances the ability of the admetal to dissociate the O–H bonds in water. When going from Pt(111) to Pt8/CeO2(111), the dissociation of water becomes a very exothermic process. The ceria-supported Pt8 appears as a fluxional system that can change geometry and charge distribution to accommodate adsorbates better. In comparison with other water-gas shift (WGS) catalysts [Cu(111), Pt(111), Cu/CeO2(111), and Au/CeO2(111)], the Pt/CeO2(111) surface has the unique property that the admetal is able to dissociate water in an efficient way. Furthermore, for the codeposition of Pt and CeOx nanoparticles on TiO2(110), we have found a transfer of O from the ceria to Pt that opens new paths for the WGS process and makes the mixed-metal oxide an extremely active catalyst for the production of hydrogen.
Ref: Bruix, A., Rodriguez, J.A., Ramirez, P.J., Senanayake, S.D., Evans, J., Park, J.B., Stacchiola, D., Liu, P., Hrbek, J., and Illas, F. Journal of the American Chemical Society,2012. 134(21): p. 8968-8974. DOI: 10.1021/ja302070k




