- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Catalysis: Reactivity and Structure
In situ/operando studies for the production of hydrogen through the water-gas shift on metal oxide catalysts
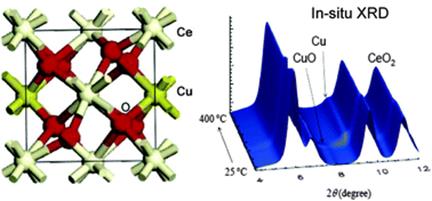 In
this perspective article, we show how a series of in situ
techniques {X-ray diffraction (XRD), pair-distribution-function analysis
(PDF), X-ray absorption fine structure (XAFS), environmental
transmission electron microscopy (ETEM), infrared spectroscopy (IR),
ambient-pressure X-ray photoelectron spectroscopy (AP-XPS)} can be
combined to perform detailed studies of the structural, electronic and
chemical properties of metal oxide catalysts used for the production of
hydrogen through the water-gas shift reaction (WGS, CO + H2O -> H-2 +
CO2). Under reaction conditions most WGS catalysts undergo chemical
transformations that drastically modify their composition with respect
to that obtained during the synthesis process. Experiments of
time-resolved in situ XRD, XAFS, and PDF indicate that the active phase
of catalysts which combine Cu, Au or Pt with oxides such as ZnO, CeO2,
TiO2, CeOx/TiO2 and Fe2O3 essentially involves nanoparticles of the
reduced noble metals. The oxide support undergoes partial reduction and
is not a simple spectator, facilitating the dissociation of water and in
some cases modifying the chemical properties of the supported metal.
Therefore, to optimize the performance of these catalysts one must take
into consideration the properties of the metal and oxide phases. IR and
AP-XPS have been used to study the reaction mechanism for the WGS on
metal oxide catalysts. Data of IR spectroscopy indicate that formate
species are not necessarily involved in the main reaction path for the
water-gas shift on Cu-, Au- and Pt-based catalysts. Thus, a pure redox
mechanism or associative mechanisms that involve either carbonate-like
(CO3, HCO3) or carboxyl (HOCO) species should be considered. In the last
two decades, there have been tremendous advances in our ability to study
catalytic materials under reaction conditions and we are moving towards
the major goal of fully understanding how the active sites for the
production of hydrogen through the WGS actually work.
In
this perspective article, we show how a series of in situ
techniques {X-ray diffraction (XRD), pair-distribution-function analysis
(PDF), X-ray absorption fine structure (XAFS), environmental
transmission electron microscopy (ETEM), infrared spectroscopy (IR),
ambient-pressure X-ray photoelectron spectroscopy (AP-XPS)} can be
combined to perform detailed studies of the structural, electronic and
chemical properties of metal oxide catalysts used for the production of
hydrogen through the water-gas shift reaction (WGS, CO + H2O -> H-2 +
CO2). Under reaction conditions most WGS catalysts undergo chemical
transformations that drastically modify their composition with respect
to that obtained during the synthesis process. Experiments of
time-resolved in situ XRD, XAFS, and PDF indicate that the active phase
of catalysts which combine Cu, Au or Pt with oxides such as ZnO, CeO2,
TiO2, CeOx/TiO2 and Fe2O3 essentially involves nanoparticles of the
reduced noble metals. The oxide support undergoes partial reduction and
is not a simple spectator, facilitating the dissociation of water and in
some cases modifying the chemical properties of the supported metal.
Therefore, to optimize the performance of these catalysts one must take
into consideration the properties of the metal and oxide phases. IR and
AP-XPS have been used to study the reaction mechanism for the WGS on
metal oxide catalysts. Data of IR spectroscopy indicate that formate
species are not necessarily involved in the main reaction path for the
water-gas shift on Cu-, Au- and Pt-based catalysts. Thus, a pure redox
mechanism or associative mechanisms that involve either carbonate-like
(CO3, HCO3) or carboxyl (HOCO) species should be considered. In the last
two decades, there have been tremendous advances in our ability to study
catalytic materials under reaction conditions and we are moving towards
the major goal of fully understanding how the active sites for the
production of hydrogen through the WGS actually work.
Ref: Rodriguez, J.A., Hanson, J.C., Stacchiola, D., and Senanayake, S.D. Physical Chemistry Chemical Physics, 2013. 15(29): p. 12004-12025. DOI: 10.1039/C3CP50416F




