- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Electrochemical Energy Storage
Correlating the Structural Changes and Gas Evolution during Thermal Decomposition of Cathode Materials Using Combined Time-resolved XRD and MS
The thermal instability of charged cathode materials has been recognized as one critical factor affecting the safety of lithium ion batteries (LIBs). When in an overcharged state, cathode materials may decompose through a serious of phase transitions that are accompanied by the release of highly reactive oxygen species that can ultimately result in thermal runaway. In order to design safer cathode materials that do not liberate oxygen species when in charged states, it is critical to better understand the thermal decomposition mechanism of charged cathode materials, including the phase transitions that occur, and the gas evolution behavior. Our group has developed a new in situ technique that combines time resolved x-ray diffraction (TR-XRD) and mass spectroscopy (MS) for these studies. This technique allows direct correlation between observed structural changes and gas evolution that occurs during thermal decomposition of charged cathode materials including layer structured, spinel, and phosphate materials.
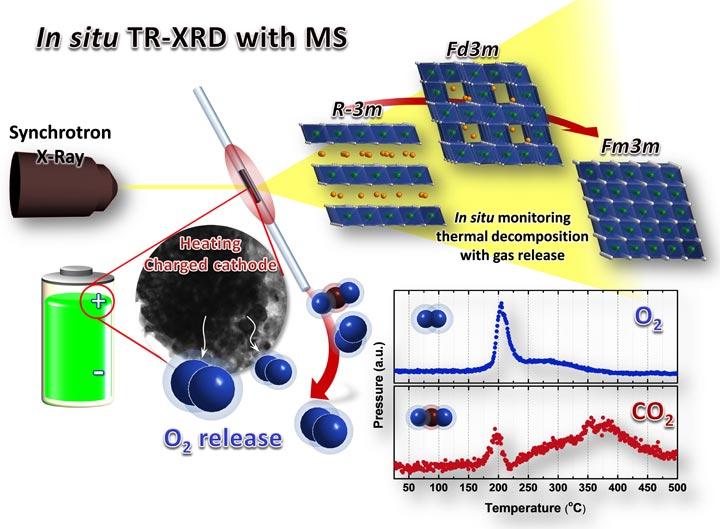
“Correlating structural changes and gas evolution during the thermal decomposition of charged LixNi0.8Co0.15Al0.05O2 cathode materials,” S. Bak, K.W. Nam, W. Chang, X.Yu, E. Hu, S. Hwang, E.A. Stach, K.B. Kim, K.Y. Chung, X.-Q. Yang, Chem. Mater. (2013), 25, 337.




